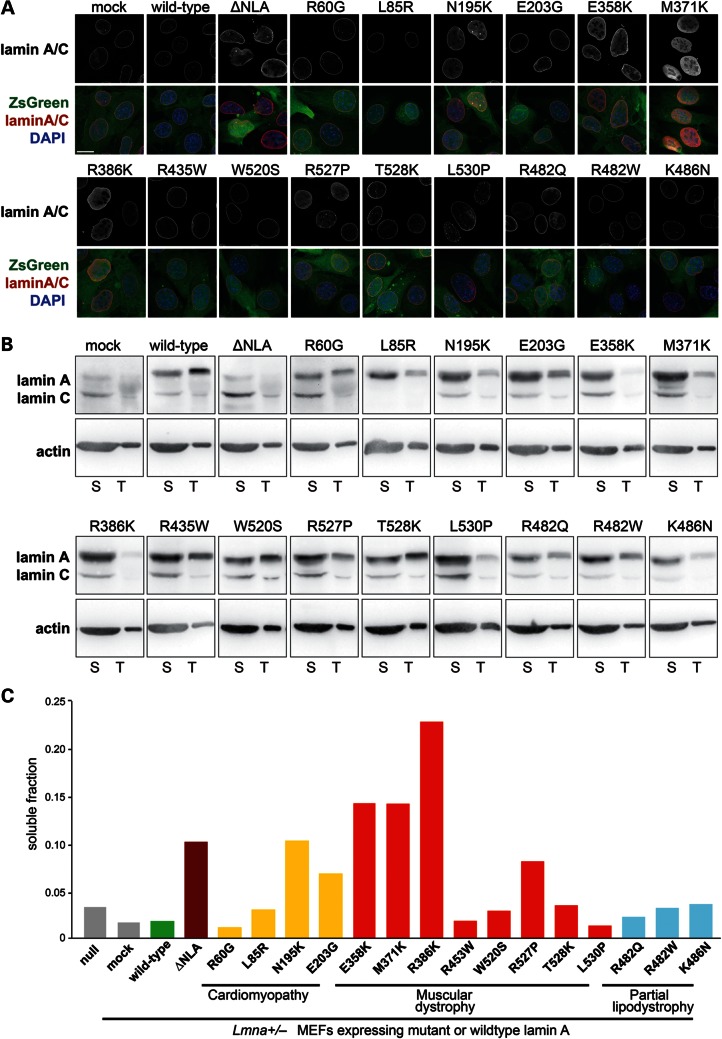
Figure 2.
Myopathic lamin A mutations that cause defects in nuclear stability have increased nucleoplasmic distribution and are more soluble. (A) Immunofluorescence staining for lamin A/C in Lmna+/− fibroblasts stably expressing the empty vector (mock), wild-type lamin A, head-truncated ΔNLA or disease-specific lamin A mutations (upper panels) and overlaid with the ZsGreen signal and DAPI chromatin staining (lower panels). Cells expressing the ΔNLA, N195K, E358K, M371K and R386K mutations have increased nucleoplasmic localization of lamin A/C. Scale bar: 20 µm. (B) Soluble lamin A protein fraction (S) versus total lamin A and C levels in Lmna+/− fibroblasts stably expressing the empty vector (mock), wild-type lamin A, head-truncated ΔNLA or disease-specific lamin A mutations, as detected by western analysis. The soluble fraction contains lamin protein that is not incorporated into the nuclear lamina, and was therefore extracted by treatment of cells with mild detergent. Note that only 1/30 of the total lamin A fraction, compared with the soluble fraction, was loaded on the gel. Similar protein levels were loaded, as reflected by actin staining. Since different amount of cells were loaded onto gels for each cell line, a direct comparison of amounts of soluble or total Lamin A fraction between cell lines is not possible, and it is the ratio between soluble to total protein that is used for the interpretation of the results. Extraction was performed three independent times; one representative panel is shown. (C) Quantification of the soluble lamin A fraction of the cells analyzed in (B), indicating that ΔNLA and the disease-specific lamin variants N195K, E358K, M371K and R386K are more soluble than wild-type lamin A and other lamin variants.