Table 1.
Optimization of Reaction Conditions
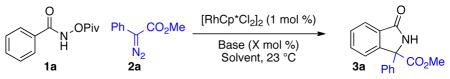
| |||
|---|---|---|---|
| entrya | base | solvent | yield (%)b |
| 1 | CsOPiv (200 mol %) | MeOH (0.5 M) | 24 |
| 2 | CsOPiv (200 mol %) | EtOH (0.5 M) | 33 |
| 3 | CsOPiv (200 mol %) | TFE (0.5 M) | 59 |
| 4 | CsOPiv (200 mol %) | MeCN (0.5 M) | 78 |
| 5 | CsOAc (200 mol %) | MeCN (0.5 M) | 80 |
| 6 | CsOAc (20 mol %) | MeCN (0.1 M) | 81c |
| 7 | - | MeCN (0.1 M) | 0 |
| 8d | CsOAc (20 mol %) | MeCN (0.1 M) | 75c |
Standard Conditions: 1a (1 equiv), 2a (1 equiv), [RhCp*Cl2]2 (1 mol %).
Yield determined by HPLC.
Isolated Yield.
Catalyst loading (0.4 mol %)
