Figure 5.
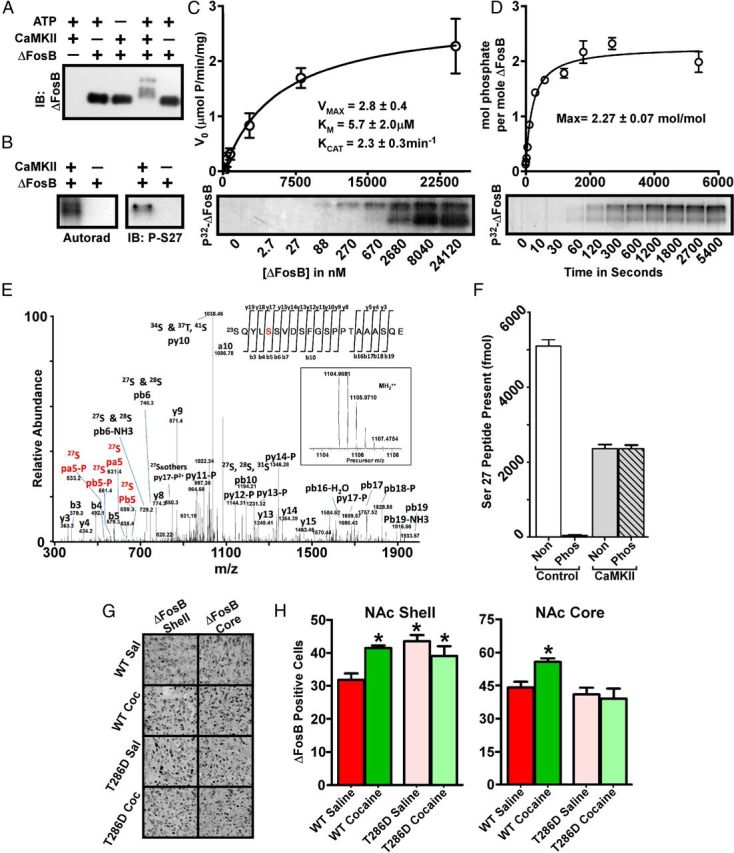
ΔFosB is a potent substrate for CaMKIIα. A, Western blotting shows an ATP-dependent multi-band shift in electrophoretic mobility of ΔFosB after exposure to CaMKIIα. IB, Immunoblot. B, Autoradiogram reveals a CaMKII-dependent incorporation of radiolabeled phosphate into ΔFosB (left), and a ΔFosB Ser27 phospho-specific antibody shows phosphorylation of this site by CaMKII (right). Analyses reveal robust kinase kinetics (C) and incorporation of multiple phosphates into ΔFosB by CaMKII (D). E, The precursor (inset) and fragment spectra of a TiO2 enriched phosphopeptide detected from ΔFosB after in vitro phosphorylation by CaMKII. After using both trypsin and GluC digestion and enrichment of the phosphopeptide samples by TiO2, analysis reveals phosphorylation of Ser27 as well as of several other sites not characterized further here. F, MRM analysis of ΔFosB phosphorylated in vitro by CaMKIIα reveals that Ser27 is a potent CaMKII substrate. Non, Nonphosphorylated peptide; Phos, phosphorylated peptide. G, Immunohistochemical analysis reveals increased ΔFosB in both the NAc shell and core of adult male wild-type (WT) mice exposed to chronic cocaine. Littermates overexpressing a constitutively active form of CaMKIIα show basal elevation in ΔFosB in the NAc shell only and show no effect of cocaine on ΔFosB levels in either region; quantified in H (n = 9–10; *p < 0.05, one-way ANOVA).
