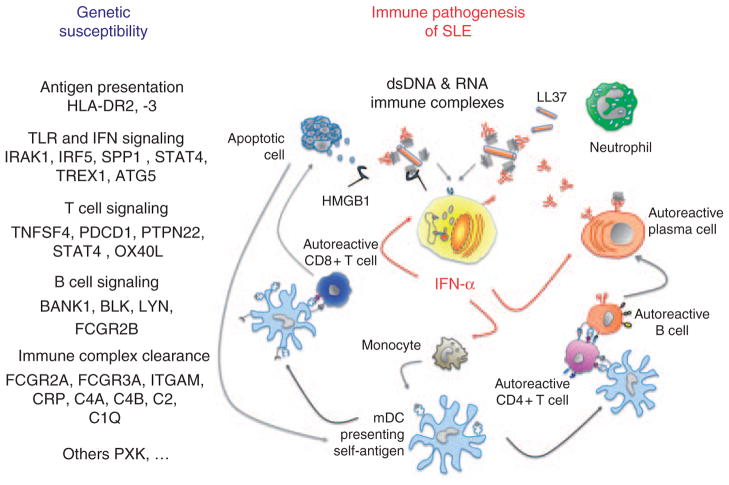
Figure 1.
The IFN-α signature of systemic lupus erythematosus (SLE). Genetic susceptibility to SLE includes genes involved in immune complex clearance, the stimulation of IFN-α production and IFN-α signaling as well as antigen presentation and B- and T-cell signaling contributing to immune pathogenesis of SLE as shown in the right part of this figure. Nucleic acids can act as endogenous triggers of IFN-α production in pDC. Molecules such as LL37 and HMGB1 not only protect self-nucleic acids from extracellular degradation, but are also involved in their uptake through lipid rafts and/or receptors such as RAGE into endosomal TLR7/9 compartments to stimulate IFN-α production. Moreover, immune complexes can bind to FcgRIIa (CD32) on pDC and thereby gain access to endosomes by receptor-mediated endocytosis. IFN-α induces and maintains the generation of mature DCs, which expand and activate rather than delete autoreactive T cells. The latter contribute to tissue damage yielding large numbers of nucleosomes, which can be captured by mature DCs, further amplifying the autoreactive process. Together with IL-6 IFN-α promotes plasmablasts to develop into antibody-secreting plasma cells. Also BLyS/BAFF is induced by IFN-α and contributes to the survival of mature, peripheral B cells. Moreover, IFNα upregulates the expression of IRF7 in pDC, and of TLR7 and IRF7 in mDC and monocytes, thereby increasing the responsiveness to DNA and/or RNA-containing immune complexes with further augmentation of IFN-α synthesis. For a color version of this figure, see online version of this article.