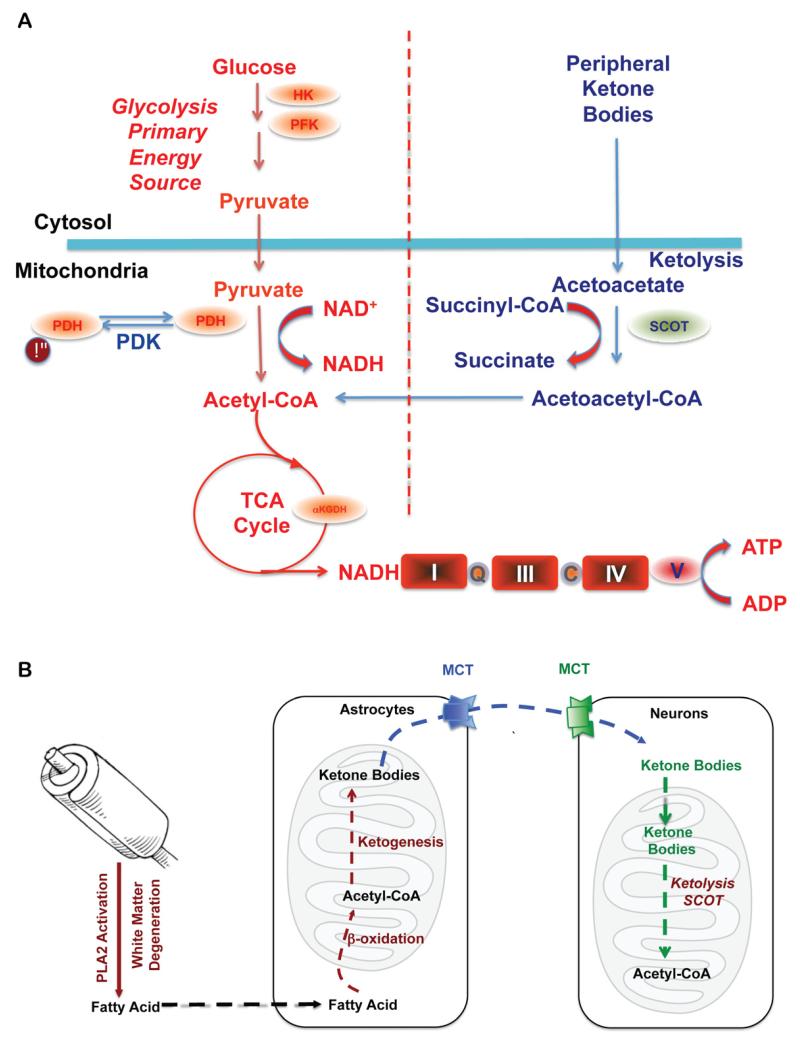
Figure 1. Bioenergetic substrate and catalytic compensatory adaptations to sustain metabolic demand of the brain.
A. Compensatory bioenergetic adaptation I: Glucose, the primary fuel source of brain metabolism, is converted via glycolysis to pyruvate which is further converted into acetyl-CoA to initiate and sustain the TCA cycle. Under metabolically challenging conditions (i.e. starvation, aging and neurodegeneration) neurons can utilize peripheral ketone bodies (β-hydroxybutyrate and acetoacetate generated by the liver) through ketolysis to generate acetyl-CoA. B. Compensatory bioenergetic adaptation II: local consumption of white matter for bioenergetics. With disease progression, peripheral ketone bodies are exhausted and the brain has to consume local white matter for energy production. Degradation of white matter via activation of PLA2 generates fatty acids that are further metabolized into acetyl-CoA through β-oxidation in the astrocytes. Acetyl-CoA is further converted into ketone bodies and transported into neurons by monocarboxylate transporters (MCTs) where ketone bodies are converted back into acetyl-CoA by SCOT and other important enzymes in ketolysis and further utilized towards ATP generation.