Figure 1. NLRP12 attenuates the development of experimental colitis.
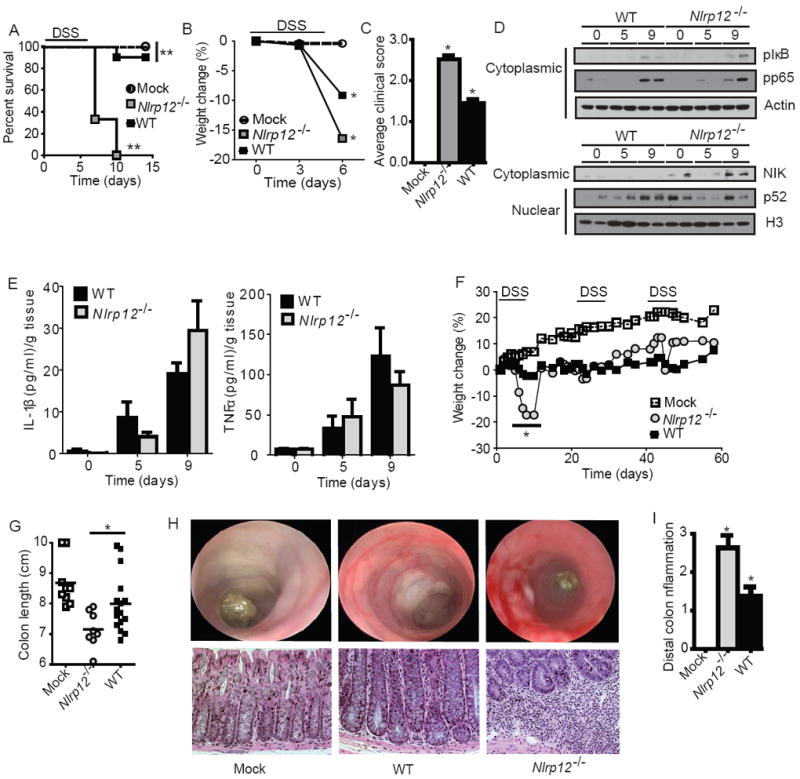
(A-E) Wild-type and Nlrp12-/- mice were challenged with 5% dextran sulfate sodium (DSS) for 5 days and disease progression was assessed daily. (A) Survival and (B) weight loss in Nlrp12-/- and wild-type mice. Due to increased mortality in the Nlrp12-/- mice, weight assessments were halted on Day 6. (C) Composite clinical scores reflecting weight loss, stool consistency and the presence of blood in the stool and/or rectum. (D) pIκBα, pp65 and NIK levels were evaluated in colons harvested from wild type and Nlrp12-/- mice (2 individual mice are shown per time point and all samples were run together on the same gel) prior to the initiation of acute colitis (Day 0) and 9 Days post-colitis initiation. (E) Colon amounts of IL-1β and TNFα were assessed by ELISA from organ culture supernatants. (A-E) WT mock, n = 4; DSS-treated WT, n = 10; Nlrp12-/-, n = 8. (F-I) Mice were treated with 3 rounds of 2.5% DSS for 5 days, followed by 2 weeks of recovery to assess recurring colitis. (F) Weight loss was assessed throughout the recurring DSS model. (G) Colon length from Nlrp12-/- and wild type mice. (H) Disease progression was assessed immediately prior to the third round of DSS (Day 37) via high resolution endoscopy. High resolution endoscopy was performed on 3 animals from each group. Histopathology revealed a considerable amount of crypt loss and immune cell infiltration in Nlrp12-/- mice compared to wild type mice. (I) Histopathology scoring revealed a significant increase in distal colon inflammation in the DSS treated Nlrp12-/- mice compared to the wild type animals. (F-I) WT mock, n = 9; DSS-treated WT, n = 16; Nlrp12-/-, n = 8. Data shown are representative of at least three independent experiments and depict the mean ± SEM. The symbols * and ** indicate P < 0.05 and P < 0.01, respectively, between the DSS-treated WT and Nlrp12-/- mice.
