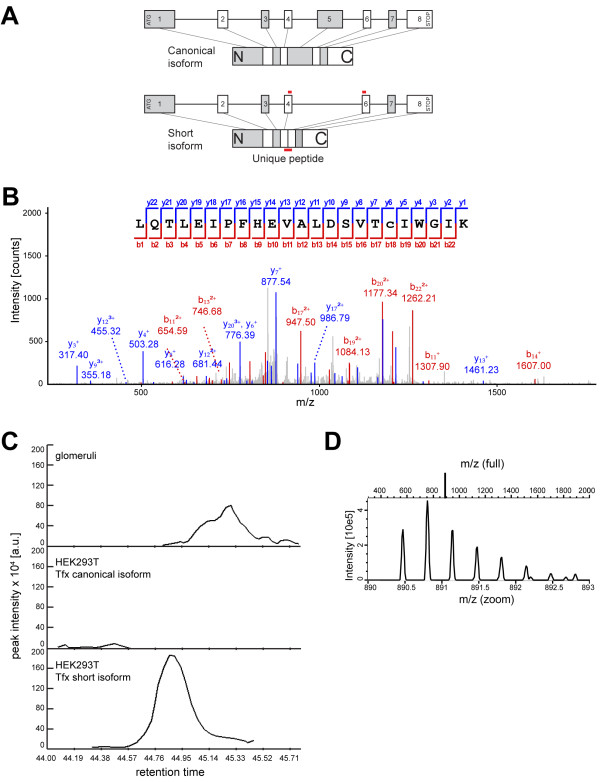
Figure 2.
Mass spectrometry proves the presence of the podocin short isoform in human kidney. A) Schematic representation of the intron/exon structure of podocin and the resulting protein. The shorter isoform lacks exon 5. To prove the existence of the shorter isoform, we searched for a unique peptide expressed from the joined ends of exon 4 and 6. Tryptic peptides extracted from a SDS-PAGE gel area corresponding to the shorter isoform of podocin were analysed by nanoLC-ESI-MS/MS. B) MS2 spectrum of the isoform specific peptide with the sequence LQTLEIPFHEVALDSVTcIWGIK (m/z = 890.47). (The non-capitalized letter “c” denotes carbamidomethylation). The peptide was unambiguously identified in protein digest of HEK 293 T cells expressing the short isoform. C) Extracted ion chromatogram of the MS1 precursor masses of the isoform specific peptide from human glomeruli, and HEK293T cells transfected with the canonical or the short podocin isoform at the time of peptide identification. The chromatogram reveals the presence of the same mass in human glomeruli at a very similar elution time (m/z = 890.47). D) MS1 isotope pattern of the respective mass of the HEK293T sample transfected with the short isoform. The isotope pattern is consistent with a triply charged peptide.