Abstract
Objective
We examined associations of perinatal and 3-year leptin with weight gain and adiposity through 7 years.
Design and Methods
In Project Viva, we assessed plasma leptin from mothers at 26–28 weeks’ gestation (n=893), umbilical cord vein at delivery (n=540), and children at 3 years (n=510) in relation to body mass index (BMI) z-score, waist circumference, skinfold thicknesses, and dual X-ray absorptiometry body fat.
Results
50.1% of children were male and 29.5% non-white. Mean(SD) maternal, cord, and age 3 leptin concentrations were 22.9(14.2), 8.8(6.4), and 1.8(1.7) ng/mL, respectively, and 3- and 7-year BMI z-scores were 0.46(1.00) and 0.35(0.97), respectively. After adjusting for parental and child characteristics, higher maternal and cord leptin was associated with less 3- year adiposity. For example, mean 3-year BMI z-score was 0.5 lower (95%CI:−0.7,−0.2; p-trend=0.003) among children whose mothers’ leptin concentrations were in the top vs. bottom quintile. In contrast, higher age 3 leptin was associated with greater weight gain and adiposity through age 7 [e.g., change in BMI z-score from 3 to 7 years was 0.2 units (95%CI:−0.0,0.4; p-trend=0.05)].
Conclusions
Higher perinatal leptin was associated with lower 3-year adiposity, whereas higher age 3 leptin was associated with greater weight gain and adiposity by 7 years.
Keywords: leptin, body mass index (BMI), children
Introduction
The major functions of leptin, a peptide hormone primarily produced by adipocytes, are to suppress appetite and stimulate metabolism. (1) Research in rodent models suggests a critical period in early life in which leptin exposure matures hypothalamic neurons responsible for appetite balance, thereby moderating subsequent energy balance and the trajectory of weight gain. (2) Administering leptin to rats during late pregnancy and lactation decreases susceptibility of male offspring to high-fat-diet-induced weight gain and insulin resistance, (3) and neonatal leptin treatment reverses the adipogenic effects of prenatal undernutrition. (4) However, later in life, some animals experience leptin tolerance, which is the inability of higher leptin concentrations to regulate energy homeostasis; some may use the terms “leptin resistance” or “decreased leptin sensitivity.” At that point, the critical window of exposure has closed. (5, 6) For example, leptin exposure in the early postnatal period prevented obesity development in leptin-deficient ob/ob mice, but exposure later in life did not. (7) Development of leptin tolerance may be due to several factors including impaired transport of leptin across the blood-brain barrier, impaired negative feedback mechanisms, stress in the endoplasmic reticulum and a saturable intracellular leptin signaling system. (8, 9, 10)
In humans, a period of leptin sensitivity also appears to exist early in life. The placenta pumps large amounts of leptin into maternal, and to a lesser extent, fetal circulation, which may act as a stimulus to maturing fetal appetite and thus appropriate weight regulation. (11) We previously reported that higher leptin in venous umbilical cord blood at delivery predicts slower child gain in weight-for-length in the first 6 months of life and lower body mass index (BMI) z-score in 3-year-old children; (12, 13) other studies showed similar associations. (14, 15, 16, 17) In contrast, in some studies in older children and adolescents, particularly among those who are overweight or obese, higher leptin predicted greater subsequent weight gain, suggesting tolerance to leptin’s appetite-regulating effects has already developed. (18, 19, 20) For example, in a study of normal-weight and overweight American children age 6–12 years, leptin at baseline was associated with greater BMI (n=197) and DXA fat mass (n=149) after an average follow-up of 4.4 years. (20) In adults, administration of recombinant leptin does lead to some weight loss, but supra-physiologic doses are required and weight loss is not substantial. (21) Leptin tolerance appears to be a key factor that makes sustained weight loss extremely challenging in overweight and obese individuals.
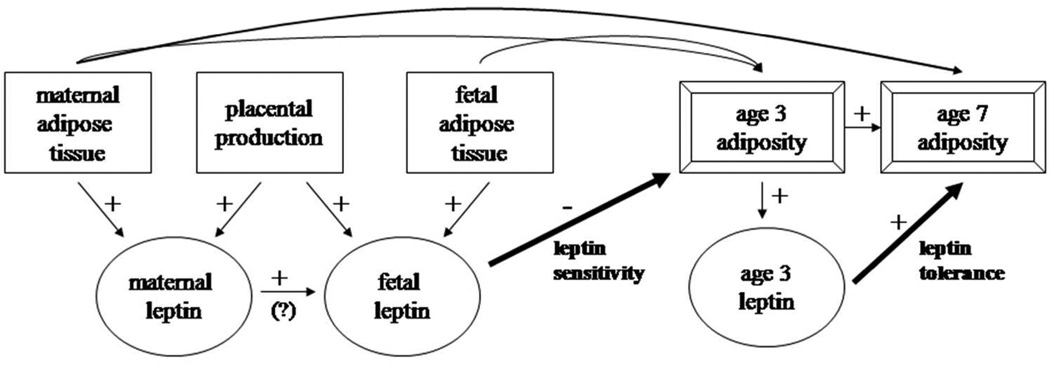
This evidence suggests that perinatal exposure to leptin reduces appetite and subsequent weight gain, whereas owing to tolerance, later exposure to leptin does not have the same weight-lowering effects (Figure 1). No previous study has examined the development of leptin tolerance longitudinally within the same cohort. The timing of any reversal in leptin’s effects is unknown and it is not clear whether such a reversal is present in normal-weight as well as overweight or obese children. Therefore, we examined leptin from mothers during pregnancy, from the umbilical vein at delivery, and from children at 3 years in relation to child weight gain and adiposity at ages 3 through 7. Based on animal experimental studies and our previous work, we hypothesized that after adjustment for maternal and fetal adiposity, maternal and cord blood leptin would be inversely associated with child adiposity, but that by age 3, the leptin-adiposity association would change to a positive direction.
Figure 1.
Conceptual model of how maternal, fetal, and child leptin affect adiposity in childhood. We hypothesize that perinatally, leptin acts on appetite-regulating pathways during a period of leptin sensitivity to decrease subsequent excess weight gain and adiposity. In contrast, by early childhood leptin tolerance, or reduced leptin sensitivity, is present, resulting in direct associations of leptin with concurrent and subsequent adiposity. Arrows indicate known or possible associations. The relative strengths of these associations vary.
Methods and Procedures
Study Population
Participants were from Project Viva, an ongoing prospective cohort study of pre- and perinatal influences on maternal, fetal, and child health. In 1999–2002 we recruited pregnant women at in-person visits in the first trimester of pregnancy from 8 obstetric offices of Harvard Vanguard Medical Associations, a multi-site group practice in eastern Massachusetts, as described previously. (22) Mother-child in-person visits occurred at 6 months, 3 years, and 7 years. Annually we collected self-reported questionnaire data, and throughout we obtained other data from medical records. The number of live births to study participants was 2,128. Of the 2,128 mother-child pairs, 1,829 had at least one leptin measurement and of those, 1,283 had BMI measurements at ages 3 and/or 7. We excluded one individual with implausible maternal leptin values (>100 ng/mL) and individuals missing any covariate data, leaving 1,063 mother-child pairs in this analytic sample.
We collected blood samples at in-person study visits conducted with the mothers at 26–28 weeks’ gestation (n=893), at delivery from the umbilical cord (n=540), and with the children at 3 years (n=510). The analytic samples included 839 mother-child pairs for maternal leptin and 3-year BMI, 717 for maternal leptin and 7-year BMI, 508 for cord leptin and 3-year BMI, 421 for cord leptin and 7-year BMI, and 510 for age 3 leptin and 7-year BMI.
Institutional review boards of Harvard Pilgrim Health Care, Brigham and Women’s Hospital and Beth Israel Deaconess Medical Center approved the study protocols and all mothers provided written informed consent.
Data Collection
To measure leptin, we refrigerated nonfasting venous whole blood samples immediately after collection and transferred them to the storage laboratory within 24 hours, where they were spun and blood components were separated into aliquots for storage in liquid nitrogen. We measured plasma leptin using an immunoassay (Linco Research Inc, St Charles, MO) as described previously. (23)
Our primary outcome was BMI z-score, but we also included waist circumference, sum of skinfolds, and DXA fat mass as outcomes. We obtained birthweight from hospital records. Trained research assistants measured child length within 2 days after birth and height at 3 and 7 years as described previously, (12) and weight with a calibrated scale (age 3: Seca model 881, Seca Corp, Hanover, Maryland; age 7: Tanita model TBF-300A, Tanita Corporation of America, Inc., Arlington Heights, Illinois). We calculated birthweight-for-gestational age z-score using national reference data from the United States Natality datasets (24) and age-sex-adjusted BMI z-score using the 2000 Centers for Disease Control and Prevention growth charts. (25, 26)
At ages 3 and 7, trained research assistants measured waist circumference to the nearest 0.1 cm using a Hoechstmass measuring tape (Hoechstmass Balzer GmbH, Sulzbach, Germany) and subscapular and triceps skinfolds as described previously. (12) We calculated the sum of the two skinfold thicknesses.
At age 7, research assistants also administered whole body DXA scans to the children with a Hologic model Discovery A fan-beam scanner (Hologic, Bedford, Massachusetts) with Hologic software QDR version 12.6. A single trained investigator (CEB) checked all DXA scans for positioning, movement, and artifacts, and defined body regions to assess whole body fat mass. Intrarater reliability of 30 scans was high (r=0.99).
Using questionnaires, interviews, and medical records we collected information on sociodemographic factors, lifestyle habits, and medical and reproductive history from the first trimester through childhood as described previously. (22) Mothers reported breastfeeding duration and child behavioral characteristics including television watching, sugar-sweetened beverage and fast food consumption, and sleep duration through age 7.
Statistical Analysis
After evaluating descriptive data, we conducted multivariable linear regression to evaluate child adiposity and change in adiposity according to quintiles of leptin, and calculated pvalues for trend across the quintiles. We assessed maternal and cord leptin individually in relation to child adiposity at 3 and 7 years as well as change in adiposity from 3 to 7. We also assessed child age 3 leptin in relation to adiposity at 7 years and change in adiposity from 3 to 7.
We built models by first examining unadjusted associations; then to evaluate confounding we assessed how associations changed when we added variables we considered a priori as confounders or which were associated with leptin and/or adiposity individually and/or in small groups to assess whether magnitudes of association changed. First we added maternal prepregnancy BMI and then sequentially added child BMI at the beginning of the time interval, socioeconomic characteristics, parental health characteristics, and child characteristics. We present three models: 1.) Unadjusted, 2.) adjusted for baseline BMI (maternal leptin model: mother’s prepregnancy BMI; cord model: mother’s prepregnancy BMI and infant birthweight-for-gestational age z-score; age 3 model: mother’s prepregnancy BMI and age 3 BMI z-score) and 3.) additionally adjusted for other covariates. For models with waist circumference, skinfolds, or DXA as outcomes, we also adjusted for child height at the outcome visit. We initially adjusted for maternal glucose tolerance and child diet, sleep, and television viewing as other potential confounders, but including these variables in our models did not substantially change exposure-outcome associations, so we did not include them in the final models.
We evaluated effect modification by child sex by including continuous interaction terms. For the analyses with 3-year leptin as the exposure, we additionally evaluated effect modification by attained 3-year BMI z-score.
We used a complete case approach for all analyses since there were few missing data for the covariates. We also analyzed the data using several approaches to multiple imputation that included imputing covariates only as well as covariates, exposures, and outcomes. Results were consistent with those presented here. We performed all calculations in SAS version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Among the 1,063 children in this analysis, 50.1% were male and 70.5% were white (Table 1). Mean (SD) BMI z-scores at ages 3 and 7 were 0.46(1.00) and 0.35(0.97), respectively. Spearman correlation coefficients were 0.10 (p=0.05) between maternal and cord blood leptin, 0.06 (p=0.21) between maternal and age 3 leptin, and 0.26 (p<0.0001) between cord blood and age 3 leptin. Compared with the analytic sample, the 1,065 mother-child pairs without complete exposure, outcome, and covariate data had higher leptin [mean(SD) for maternal concentration: 23.9(14.6) vs. 22.9(14.2); umbilical cord blood: 9.4(7.0) vs. 8.8(6.4); age 3: 2.5(3.0) vs. 1.8(1.7) ng/mL]; and mothers with higher prepregnancy BMI [25.1(6.0) vs. 24.7(5.2) kg/m2], younger age [31.1(5.4) vs. 32.6(4.9) years], greater smoking during pregnancy (16.0% vs. 10.1%), less education (56.1% vs. 73.0% with college degree or higher), and lower household income (54.8% vs. 66.3% with income more than $70,000). However, paternal BMI, maternal gestational weight gain, and child birthweight-for-gestational age z-score were similar in the two groups.
Table 1.
Characteristics of the 1063 participating maternal-child pairs from Project Viva.
| Variable | N | Mean (SD)/N(%) |
|---|---|---|
| Maternal/paternal characteristics | ||
| 2nd trimester leptin, ng/mL | 893 | 22.9 (14.2) |
| Age at enrollment (mean 10 weeks’ gestation), y | 1,063 | 32.6 (4.9) |
| Prepregnancy BMI, kg/m2 | 1,063 | 24.7 (5.2) |
| Paternal BMI, kg/m2 | 1,063 | 26.4 (3.8) |
| Gestational weight gain, kg | 1,063 | 15.6 (5.3) |
| Total physical activity in 2nd trimester, hrs/wk | 950 | 6.7 (6.3) |
| Total energy intake in 2nd trimester, kcal/day | 962 | 2,160 (620) |
| Smoked during pregnancy | 1,063 | 107 (10.1%) |
| Education level, at least college degree | 1,063 | 776 (73.0%) |
| Household income, more than $70,000 | 1,036 | 687 (66.3%) |
| Child Characteristics | ||
| Venous cord blood leptin, ng/mL | 540 | 8.8 (6.4) |
| Age 3 leptin, ng/mL | 604 | 1.8 (1.7) |
| Birthweight, g | 1,062 | 3,501 (545) |
| Gestational age at birth, weeks | 1,063 | 39.5 (1.8) |
| Birthweight-for-gestational-age z-score | 1,062 | 0.23 (0.94) |
| Breastfeeding duration, mo | 1,063 | 6.4 (4.6) |
| Age 3 BMI, kg/m2 | 1,003 | 16.5 (1.5) |
| Age 3 BMI z-score | 1,003 | 0.46 (1.00) |
| Age 3 waist circumference, cm | 1,001 | 51.3 (3.5) |
| Age 3 SS+TRa, mm | 970 | 16.7 (4.1) |
| Age 7 BMI, kg/m2 | 860 | 17.0 (2.7) |
| Age 7 BMI z-score | 860 | 0.35 (0.97) |
| Age 7 DXA fat mass, kg | 689 | 7.1 (3.4) |
| Age 7 waist circumference, cm | 860 | 59.4 (7.8) |
| Age 7 SS+TRa, mm | 859 | 19.3 (9.6) |
| Change in BMI z-score between ages 3 and 7 | 800 | −0.1 (0.8) |
| Change in waist circumference between ages 3 and 7, cm | 803 | 8.1 (6.2) |
| Change in SS+TRa between ages 3 and 7, mm | 779 | 2.3 (7.5) |
| Male sex | 1,063 | 532 (50.1%) |
| Race/ethnicity | ||
| White | 1,063 | 749 (70.5%) |
| Black | 1,063 | 125 (11.8%) |
| Hispanic | 1,063 | 41 (3.9%) |
| Asian | 1,063 | 34 (3.2%) |
| More than one race/Other | 1,063 | 114 (10.7%) |
SS+TR: sum of subscapular and triceps skinfold thicknesses.
In unadjusted models, higher maternal and age 3 leptin, but not cord leptin, were associated with greater child BMI z-score (Table 2). However, adjusting for maternal prepregnancy BMI and child birthweight-for-gestational age z-score revealed inverse associations of maternal and cord leptin with child BMI z-score. In comparison, adjusting for socio-demographic characteristics changed associations less. Adjusting for child 3-year BMI z-score attenuated the positive association between age 3 leptin and BMI z-score at age 7. The adjusted difference for the comparison of highest vs. lowest quintile of age 3 leptin changed from 0.5 (95%CI:0.2,0.7; p-trend<0.0001 across quintiles) when adjusted only for maternal BMI to 0.2 (95%CI:−0.0,0.4; p-trend=0.04) when additionally adjusted for child BMI z-score.
Table 2.
Associations of BMI z-score at ages 3 and 7 with quintile of plasma leptin from 2nd trimester of pregnancy, venous umbilical cord blood at delivery, and children at 3 years.a
| Difference in means vs. Q1 (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | P trend |
| Maternal leptin, median (range) | 7.4 (2.1–<11.0) | 13.8 (11.0–<16.6) | 19.8 (16.6–<24.0) | 28.6 (24.0–<33.8) | 41.4 (33.8–99.5) | |
| Age 3 BMI Z (n=839) | ||||||
| M1. Unadjusted | 0.00 | 0.0 (−0.2, 0.2) | 0.2 (−0.0, 0.4) | 0.2 (0.0, 0.5) | 0.2 (−0.0, 0.4) | 0.01 |
| M2. M1 + maternal BMI | 0.00 | −0.1 (−0.3, 0.2) | 0.1 (−0.1, 0.3) | −0.0 (−0.2, 0.2) | −0.3 (−0.5, −0.0) | 0.15 |
| M3. M2 + other covariatesb | 0.00 | −0.1 (−0.3, 0.1) | −0.1 (−0.3, 0.1) | −0.2 (−0.4, 0.1) | −0.5 (−0.7, −0.2) | 0.003 |
| Age 7 BMI Z (n=717) | ||||||
| M1. Unadjusted | 0.00 | 0.0 (−0.2, 0.2) | 0.3 (0.0, 0.5) | 0.3 (0.1, 0.5) | 0.4 (0.2, 0.6) | <0.0001 |
| M2. M1 + maternal BMI | 0.00 | −0.1 (−0.3, 0.1) | 0.1 (−0.1, 0.3) | −0.0 (−0.2, 0.2) | −0.2 (−0.4, 0.1) | 0.51 |
| M3. M2 + other covariatesb | 0.00 | −0.1 (−0.3, 0.1) | −0.0 (−0.3, 0.2) | −0.2 (−0.4, 0.0) | −0.4 (−0.6, −0.1) | 0.01 |
| Change in BMI Z between ages 3 and 7 (n=663) | ||||||
| M1. Unadjusted | 0.00 | −0.0 (−0.2, 0.1) | 0.1 (−0.1, 0.2) | 0.1 (−0.1, 0.2) | 0.2 (0.1, 0.4) | 0.01 |
| M2. M1 + maternal BMI | 0.00 | −0.1 (−0.2, 0.1) | 0.0 (−0.2, 0.2) | −0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.29 |
| M3. M2 + other covariatesb | 0.00 | −0.1 (−0.2, 0.1) | 0.0 (−0.2, 0.2) | −0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.44 |
| Cord leptin, median (range) | 2.2 (0.5–<3.5) | 4.4 (3.5–<5.5) | 7.2 (5.5–<8.7) | 10.7 (8.7–<14.3) | 18.8 (14.3–40.0) | |
| Age 3 BMI Z (n=508) | ||||||
| M1. Unadjusted | 0.00 | −0.2 (−0.5, 0.1) | 0.0 (−0.3, 0.3) | 0.0 (−0.3, 0.3) | −0.2 (−0.5, 0.1) | 0.53 |
| M2. M1 + maternal BMI + BW/GA-zc |
0.00 | −0.2 (−0.5, 0.1) | −0.1 (−0.4, 0.2) | −0.2 (−0.4, 0.1) | −0.5 (−0.8, −0.2) | 0.004 |
| M3. M2 + other covariatesb | 0.00 | −0.2 (−0.5, 0.1) | −0.1 (−0.4, 0.2) | −0.2 (−0.5, 0.0) | −0.5 (−0.8, −0.2) | 0.003 |
| Age 7 BMI Z (n=421) | ||||||
| M1. Unadjusted | 0.00 | −0.2 (−0.5, 0.1) | −0.1 (−0.4, 0.2) | −0.1 (−0.4, 0.2) | −0.2 (−0.5, 0.1) | 0.32 |
| M2. M1 + maternal BMI + BW/GA-zc |
0.00 | −0.2 (−0.5, 0.1) | −0.2 (−0.5, 0.1) | −0.2 (−0.5, 0.0) | −0.5 (−0.8, −0.2) | 0.004 |
| M3. M2 + other covariatesb | 0.00 | −0.2 (−0.5, 0.0) | −0.2 (−0.5, 0.0) | −0.3 (−0.6, 0.0) | −0.4 (−0.7, −0.1) | 0.02 |
| Change in BMI Z between ages 3 and 7 (n=389) | ||||||
| M1. Unadjusted | 0.00 | −0.1 (−0.3, 0.2) | −0.3 (−0.5, −0.0) | −0.1 (−0.3, 0.1) | −0.0 (−0.3, 0.2) | 0.84 |
| M2. M1 + maternal BMI + BW/GA-zc |
0.00 | −0.1 (−0.3, 0.2) | −0.2 (−0.5, 0.0) | −0.1 (−0.3, 0.2) | 0.0 (−0.2, 0.3) | 0.94 |
| M3. M2 + other covariatesb | 0.00 | −0.1 (−0.3, 0.2) | −0.3 (−0.5, −0.0) | −0.1 (−0.3, 0.2) | 0.1 (−0.2, 0.4) | 0.72 |
| Child age 3 leptin, median(range) | 0.5 (0.1–<0.8) | 1.1 (0.8–<1.2) | 1.4 (1.2–<1.7) | 2.0 (1.7–<2.5) | 3.4 (2.5–14.0) | |
| Age 7 BMI Z (n=510) | ||||||
| M1. Unadjusted | 0.00 | 0.0 (−0.3, 0.3) | 0.2 (−0.0, 0.5) | 0.3 (0.0, 0.5) | 0.6 (0.3, 0.8) | <0.0001 |
| M2. M1 + maternal BMI + child BMI z at 3y |
0.00 | −0.1 (−0.3, 0.1) | −0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.2 (−0.0, 0.4) | 0.04 |
| M3. M2 + other covariatesb | 0.00 | −0.0 (−0.2, 0.2) | 0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.2 (−0.0, 0.4) | 0.05 |
| Change in BMI Z between ages 3 age 7 (n=510) | ||||||
| M1. Unadjusted | 0.00 | −0.1 (−0.3, 0.2) | −0.1 (−0.3, 0.1) | 0.0 (−0.2, 0.2) | −0.0 (−0.2, 0.2) | 0.88 |
| M2. M1 + maternal BMI + child BMI z at 3y |
0.00 | −0.1 (−0.3, 0.1) | −0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.2 (−0.0, 0.4) | 0.04 |
| M3. M2 + other covariatesb | 0.00 | −0.0 (−0.2, 0.2) | 0.0 (−0.2, 0.2) | 0.1 (−0.1, 0.3) | 0.2 (−0.0, 0.4) | 0.05 |
Data from maternal-child pairs in Project Viva. Change in BMI z-score from ages 3 to 7 also assessed as an outcome. Bold indicates p<0.05 comparing the top quintile to the bottom quintile in the final model.
Other covariates are as follows: Household income, maternal age, education level, smoking status, and gestational weight gain; paternal BMI; and child sex, race/ethnicity, breastfeeding duration, and exact age at exposure and outcome measurements. Change in BMI z-score outcome adjusted for age at both 3 year and 7 year visits.
BW/GA-z: birth weight-for-gestational-age z-score.
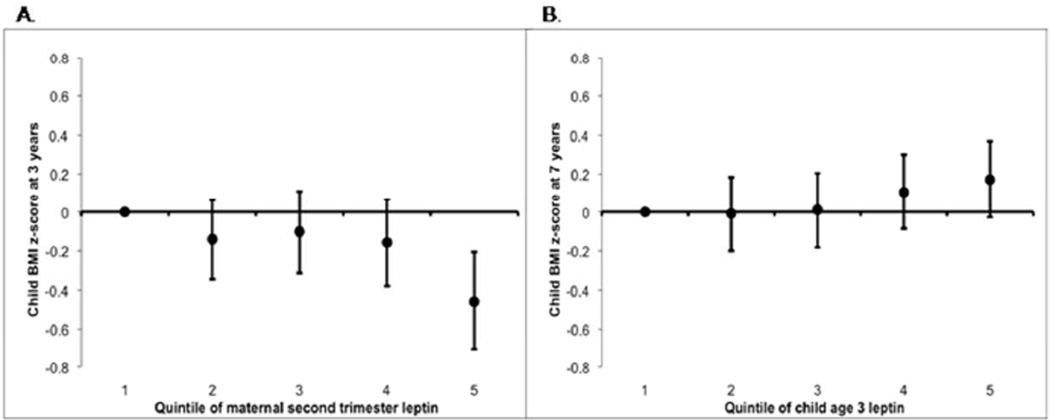
In fully adjusted models, higher maternal leptin and cord leptin were associated with lower 3-year BMI z-score, waist circumference, and sum of skinfolds, with the exception of maternal leptin and sum of skinfolds (Tables 2 and 3). For example, second trimester maternal leptin in the highest (vs. lowest) quintile was associated with child BMI z-scores 0.5 units lower (95%CI:−0.7,−0.2; p-trend=0.003 across quintiles; Figure 2). At age 7, results were suggestive of a similar inverse association but less consistent. Maternal and cord blood leptin were each inversely associated with 7-year BMI z-score [e.g., 0.4 units lower (95%CI:−0.6,−0.1; p-trend=0.01) for highest vs. lowest quintile of maternal leptin] but were not consistently associated with waist circumference, DXA fat, or sum of skinfolds at age 7, or change in adiposity from ages 3 to 7 (Table 3).
Table 3.
Multivariable associations of leptin concentrations with adiposity measures at ages 3 and 7 years, and with change in BMI z-score from 3 to 7 years. Estimates show association for highest vs. lowest quintiles of plasma leptin concentration.a
| Exposure | |||
|---|---|---|---|
| Maternal leptin | Cord leptin | Child age 3 leptin | |
| Difference in means: Q5 vs. Q1 (95% CI) | |||
| Age 3 outcomes | |||
| BMI Z | −0.5 (−0.7, −0.2) | −0.5 (−0.8, −0.2) | - |
| Waist circumference (cm) | −1.3 (−2.1, −0.5) | −1.4 (−2.3, −0.4) | - |
| SS+TRb (mm) | −0.8 (−1.8, 0.3) | −1.4 (−2.7, −0.1) | - |
| Age 7 outcomes | |||
| BMI Z | −0.4 (−0.6, −0.1) | −0.4 (−0.7, −0.1) | 0.2 (−0.0, 0.4) |
| Waist circumference (cm) | −2.1 (−3.8, −0.4) | 0.1 (−2.0, 2.1) | 2.0 (0.6, 3.5) |
| SS+TRb (mm) | 0.2 (−2.1, 2.5) | 1.1 (−1.5, 3.7) | 4.5 (2.5, 6.4) |
| DXA fat mass (kg) | 0.2 (−0.7, 1.0) | 0.3 (−0.7, 1.3) | 1.6 (0.9, 2.3) |
| Change between ages 3 and 7 | |||
| BMI Z | 0.1 (−0.1, 0.3) | 0.1 (−0.2, 0.4) | 0.2 (−0.0, 0.4) |
| Waist circumferences (cm) | −1.1 (−2.7, 0.4) | 0.3 (−1.5, 2.1) | 2.3 (0.9, 3.7) |
| SS+TRb (mm) | −0.0 (−1.9, 1.9) | 0.7 (−1.6, 3.0) | 3.7 (1.8, 5.6) |
Data from maternal-child pairs in Project Viva. Covariates are the same as in Model 3 from Table 2. Waist circumference, change in waist circumference, SS+TR, change in SS+TR, and DXA models additionally adjusted for child height. Change outcomes adjusted for age at both 3 year and 7 year visits. Bold indicates p<0.05.
SS+TR: sum of subscapular and triceps skinfold thicknesses.
Figure 2.
Maternal 2nd trimester leptin concentration in relation to child BMI z-score at age 3 (Panel A; p for trend=0.01), and child age 3 leptin concentration in relation to child BMI z-score at age 7 (Panel B; p for trend=0.05). Adjusted for maternal prepregnancy BMI, child BMI z-score at age 3 (Panel B only), household income, maternal age, education level, smoking status, and gestational weight gain; paternal BMI; and child sex, race/ethnicity, breastfeeding duration, and exact age at exposure and outcome measurements.
In multivariable models, higher age 3 leptin was associated with increased adiposity by age 7 as measured by BMI z-score, waist circumference, sum of skinfolds, and DXA. For example, children in the highest (vs. lowest) quintile of 3-year leptin had higher mean BMI z-score (0.2 units [95%CI:−0.0,0.4; p-trend=0.05]; Figure 2), waist circumference [2.0 centimeters (95%CI:0.6,3.5; p-trend=0.004)], sum of skinfolds [4.5 millimeters (95%CI:2.5,6.4, p-trend< 0.0001)], and DXA fat mass [1.6 kg (95%CI:0.9,2.3; p-trend<0.0001)]. Higher 3-year leptin was also associated with greater change in adiposity between ages 3 and 7. For example, change in BMI z-score from 3 to 7 years was 0.2 kg/m2 higher (95%CI:−0.0,0.4; p-trend=0.05) among children whose 3-year leptin concentrations were in the top vs. bottom quintile.
Results were similar when we restricted the analysis to those with data on all three leptin measurements, BMI at ages 3 and 7, and all covariates (n=183).
We did not observe effect modification by sex (e.g., interaction p=0.70 for maternal leptin and 3-year BMI z-score) or modification of the associations of 3-year leptin with 7-year adiposity (or with change in adiposity from 3 to 7) by child BMI z-score at age 3 (e.g., interaction p=0.28 for outcome of BMI z-score at age 7).
Discussion
Among mother-child pairs in a single cohort study, we found that higher maternal second trimester and venous umbilical cord blood leptin concentrations were associated with less adiposity at 3 years, whereas higher age 3 leptin was associated with greater weight gain and adiposity through age 7. This study extends our previous finding that high cord blood leptin predicts lower initial weight gain and adiposity. Most importantly, we found a positive association between age 3 leptin and weight gain and adiposity through age 7. This finding supports our hypothesis of a critical perinatal period of leptin sensitivity followed by reduced leptin sensitivity in early childhood.
Although we could not measure it directly, we used epidemiologic methods to estimate fetal exposure to leptin in mid- and late-pregnancy. By adjusting for maternal BMI and birthweight-for-gestational age we sought to assess leptin exposure independent of concurrent maternal or fetal adiposity, which could be related to child adiposity through other mechanisms. For this reason, while the unadjusted associations between maternal leptin and child BMI z-score were positive, adjustment for maternal fat mass rendered the associations inverse. Analogously, cord leptin was not associated with child 3- and 7-year BMI z-score in unadjusted models, but adjustment for maternal and infant fat mass caused that association to be inverse. We speculate that the placenta plays an important role in fetal leptin exposure. Together with the concordance of results for maternal and cord leptin, our findings suggest that the observed inverse associations between perinatal leptin and child adiposity are largely from fetal leptin exposure, and that associations are consistent in mid- to late-pregnancy.
Our finding that maternal and cord blood leptin was inversely associated with child 3-year adiposity extends our previous findings in the same cohort that higher cord blood leptin is associated with smaller change in weight-for-height from birth through 6 months and lower BMI z-score at age 3. (12) Our cord blood findings are consistent with results in other cohorts in infants through adolescents. (14, 15, 16, 17) For example, in a study of 197 infants in the United Kingdom, higher cord blood leptin was associated with less rapid weight gain from birth through 24 months. (15)
Leptin at 3 years was positively associated with weight gain over the ensuing four years, a novel finding in such a young age group. Adjustment for 3-year BMI z-score attenuated the association between age 3 leptin and age 7 adiposity, indicating that the unadjusted association was driven, at least in part, by baseline adiposity. However, even after adjustment, a positive association between leptin and adiposity was present. This finding suggests tolerance to leptin’s weight-regulating effects, which is supported by intervention studies showing that leptin administration to overweight and obese individuals has little to no effect on adiposity. (21, 27, 28, 29) However, individuals with congenital leptin deficiency gain weight rapidly and become obese, but return to normal weight when administered exogenous leptin, suggesting that leptin itself is necessary to produce leptin tolerance. (30, 31) The children in our study were younger than those in previous studies that found a positive association between leptin and weight gain, (18, 19, 20) and were largely of normal weight. Together with our findings of no effect modification by BMI, these findings suggest that leptin tolerance begins in the first 3 years of life, and applies to children with both normal and high BMI z-scores. However, two previous studies in healthy groups of children and adolescents older than ours (>6 years old at baseline) found that leptin was inversely associated with weight gain. (16, 17) One possible explanation for the different findings is that those studies did not fully adjust for potential confounders of the associations of leptin with adiposity. In addition, leptin concentrations and sensitivity may change during puberty, and they may vary across populations with a different make-up of social, ethnic, and adiposity characteristics.
Our findings are also consistent with animal research suggesting early-life programming of leptin sensitivity but later leptin insensitivity. (3, 4, 7) Yuen et al. reported that infusion of leptin into the fetal sheep circulation caused changes in lipid storage characteristics and leptin synthetic capacity. (32) Leptin administration to male rats from postnatal days 3–13 slowed weight gain in those that were undernourished in utero, and increased weight gain in those that were adequately nourished. (33) Neonatal leptin administration effectively abolished the lifetime dysmetabolism otherwise caused by exposing a pregnant dam to a low-energy diet. (33) However, other studies in rodents found evidence of decreased leptin sensitivity during adulthood. For example, rats that were undernourished during gestation did not lose weight when administered leptin as adults, (6) and obese mice lost less weight than lean mice when administered leptin. (5) Early postnatal leptin exposure prevented obesity development in leptin-deficient ob/ob mice, but later exposure did not. (7)
A period of leptin sensitivity followed by leptin tolerance has important implications for obesity prevention. Leptin production during gestation may be sensitive to external influences, in turn programming the child’s neural circuitry to determine later appetite and metabolism. Our study suggests that leptin exposure in the perinatal period may affect child metabolism and satiety signals, in turn raising the possibility that interventions to alter leptin physiology early in human development may have lasting effects on obesity prevention.
To mount preventive interventions targeting leptin physiology, more information about modifiable determinants of perinatal leptin is needed. Maternal over- or under-nutrition during pregnancy affected adipose leptin mRNA and plasma leptin concentration in sheep, (34) and diabetes in pregnant mouse dams impaired leptin sensitivity in their offspring. (35) We previously found in Project Viva that maternal energy intake and diet patterns during pregnancy were not associated with cord leptin, but that higher protein intake was weakly associated with lower cord leptin. (36)
Strengths of this study include prospectively measured exposure and outcome data, careful control of confounders, and measurement of exposures and outcomes at multiple time points in early life, allowing for observation of changes in leptin sensitivity over time.
This study has several limitations. We did not have access to tissue from the placenta, so we could not directly examine placental leptin in relation to child adiposity. Nevertheless, we obtained cord blood from the umbilical vein, which drains the placenta. Another limitation is that, as in most epidemiologic studies, we did not have access to frequently sampled leptin to assess the impact of circadian variation, nor were we able to assess changes in leptin in response to experimental conditions such as administration of other hormones. (37) Also, owing to the vicissitudes of collecting blood at delivery and from 3-year-olds, we did not have leptin at all three time points on all individuals. However, socio-demographic characteristics were similar among the three study samples, implying that the results at each time interval should be internally valid. To assess the potential for bias due to loss to follow-up, we ran analyses using multiply imputated data sets and results were similar to those presented. Nevertheless, differences between participants included in this analytic sample and the rest of the cohort may have introduced some bias to the analysis. We were unable to directly measure fat mass at the time of leptin measurement, so there may be some residual confounding by body fat. Finally, since this study is observational, it is important to interpret our findings with caution and compare our results with whole animal experiments, in vitro studies, and ultimately, randomized controlled trials, to establish causality.
In conclusion, higher maternal prenatal and cord blood leptin concentrations were associated with lower child adiposity at age 3, whereas leptin at age 3 predicted higher weight gain in the subsequent four years. These findings are consistent with the hypothesis of a perinatal period of leptin sensitivity followed by a subsequent decrease in leptin sensitivity by age 3. We speculate that leptin tolerance, which makes weight loss so difficult in adulthood, may begin in early childhood, highlighting that interventions to prevent obesity may be especially effective during early, plastic stages of human development. (38)
What is already known about this subject.
Animal studies suggest a critical period of leptin action in early development to mature appetite-regulating pathways, but later leptin tolerance.
In humans, lower cord blood leptin predicts greater weight gain and adiposity in the first years of life.
Obese adults appear to be resistant to leptin’s appetite-regulating effects.
What this study adds.
Higher maternal leptin concentrations were associated with lower adiposity at 3 years.
In contrast, higher age 3 leptin was associated with greater weight gain and adiposity by 7 years.
These findings suggest that leptin tolerance is not present at birth but may begin by 3 years.
Acknowledgments
We thank the participants and research staff of Project Viva. This research was funded by grants from the National Institutes of Health (K24 HL 068041, R01 HD 034568, and T32 CA 09001). MWG conceptualized and designed the study, CEB and SLR carried out the analyses, and CEB, CSM, MDH, SLR, EV, CAZ, and MWG interpreted the data, critically reviewed the manuscript, and approved the final manuscript.
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology--emerging clinical applications. Nat Clin Pract Endocrinol Metab. 2006;2:318–327. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 2.Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301. doi: 10.1111/j.1399-0004.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Stocker C, O'Dowd J, Morton NM, et al. Modulation of susceptibility to weight gain and insulin resistance in low birthweight rats by treatment of their mothers with leptin during pregnancy and lactation. Int J Obes Relat Metab Disord. 2004;28:129–136. doi: 10.1038/sj.ijo.0802476. [DOI] [PubMed] [Google Scholar]
- 4.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, Boozer C, Blair-West J, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krechowec SO, Vickers M, Gertler A, et al. Prenatal influences on leptin sensitivity and susceptibility to diet-induced obesity. J Endocrinol. 2006;189:355–363. doi: 10.1677/joe.1.06679. [DOI] [PubMed] [Google Scholar]
- 7.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 8.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon HS, Chamberland JP, Diakopoulos KN, et al. Leptin and amylin act in an additive manner to activate overlapping signaling pathways in peripheral tissues: in vitro and ex vivo studies in humans. Diabetes Care. 2011;34:132–138. doi: 10.2337/dc10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194:1537–1545. doi: 10.1016/j.ajog.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 12.Mantzoros CS, Rifas-Shiman SL, Williams CJ, et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker M, Rifas-Shiman SL, Belfort MB, et al. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J Pediatr. 2011;158:227–233. doi: 10.1016/j.jpeds.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca VM, Sichieri R, Moreira ME, et al. Early postnatal growth in preterm infants and cord blood leptin. J Perinatol. 2004;24:751–756. doi: 10.1038/sj.jp.7211188. [DOI] [PubMed] [Google Scholar]
- 15.Ong KK, Ahmed ML, Sherriff A, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- 16.Byrnes SE, Baur LA, Bermingham M, et al. Leptin and total cholesterol as predictors of weight gain in prepubertal children. Int J Obes Relat Metab Disord. 1999;23:146-–150. doi: 10.1038/sj.ijo.0800783. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed ML, Ong KK, Morrell DJ, et al. Longitudinal study of leptin concentrations during puberty: sex differences and relationship to changes in body composition. J Clin Endocrinol Metab. 1999;84:899–905. doi: 10.1210/jcem.84.3.5559. [DOI] [PubMed] [Google Scholar]
- 18.Savoye M, Dziura J, Castle J, et al. Importance of plasma leptin in predicting future weight gain in obese children: a two-and-a-half-year longitudinal study. Int J Obes Relat Metab Disord. 2002;26:942–946. doi: 10.1038/sj.ijo.0802018. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MS, Huang TT, Figueroa-Colon R, et al. Influence of leptin on changes in body fat during growth in African American and white children. Obes Res. 2001;9:593–598. doi: 10.1038/oby.2001.78. [DOI] [PubMed] [Google Scholar]
- 20.Fleisch AF, Agarwal N, Roberts MD, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. J Clin Endocrinol Metab. 2007;92:948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 22.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 23.Mantzoros CS, Liolios AD, Tritos NA, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–186. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 24.Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 27.Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 28.Lejeune MP, Hukshorn CJ, Saris WH, et al. Effect of dietary restraint during and following pegylated recombinant leptin (PEG-OB) treatment of overweight men. Int J Obes Relat Metab Disord. 2003;27:1494–1499. doi: 10.1038/sj.ijo.0802431. [DOI] [PubMed] [Google Scholar]
- 29.Fogteloo AJ, Pijl H, Frolich M, et al. Effects of recombinant human leptin treatment as an adjunct of moderate energy restriction on body weight, resting energy expenditure and energy intake in obese humans. Diabetes Nutr Metab. 2003;16:109–114. [PubMed] [Google Scholar]
- 30.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 31.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 32.Yuen BS, Owens PC, Muhlhausler BS, et al. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB J. 2003;17:1102–1104. doi: 10.1096/fj.02-0756fje. [DOI] [PubMed] [Google Scholar]
- 33.Vickers MH, Gluckman PD, Coveny AH, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 34.Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. J Physiol. 2009;587:1145–1152. doi: 10.1113/jphysiol.2008.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology. 2011;152:4171–4179. doi: 10.1210/en.2011-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantzoros CS, Sweeney L, Williams CJ, et al. Maternal diet and cord blood leptin and adiponectin concentrations at birth. Clin Nutr. 2010;29:622–626. doi: 10.1016/j.clnu.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castracane VD, Hensen MC. Leptin. New York: Spring Science+Business Media, LLC; [Google Scholar]
- 38.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]