Figure 1. Calendar time periods for stage III colon cancer patients based on first date of 5-FU or oxaliplatin receipt (N=2800) and FDA approval history of oxaliplatin.
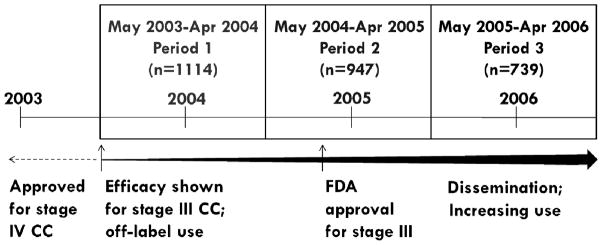
5-FU=5-Fluorouracil; FDA=Food and Drug Administration; CC=colon cancer
Efficacy results based on the MOSAIC clinical trial, presented in May 2003; FDA approval for stage III colon cancer granted in November 2004.
Time period 1 (May 2003-April 2004) was used as referent time period in the conventional PS model that adjusts for calendar time as a confounder.
