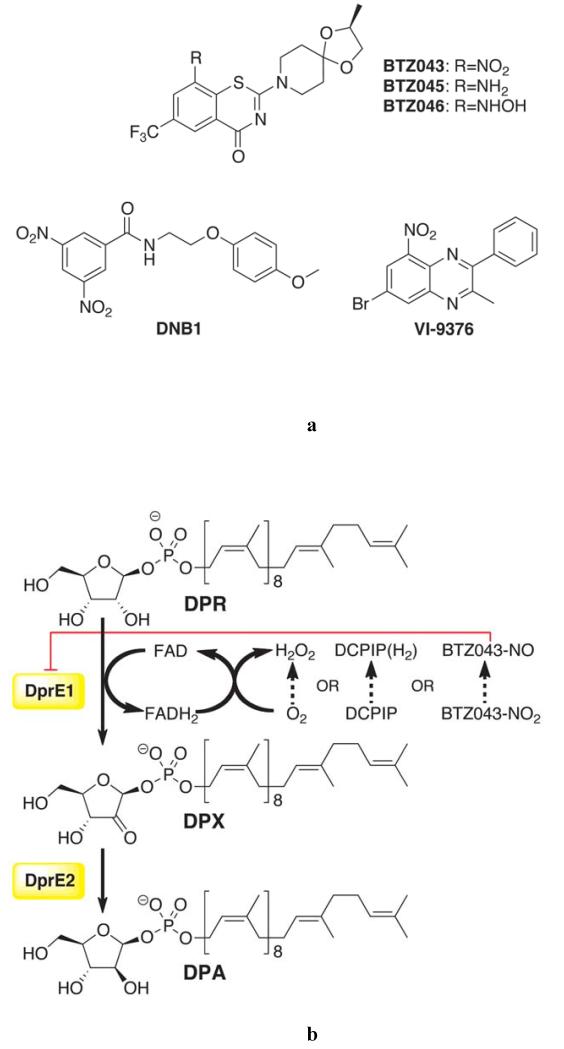
Fig. 1.
Inhibitors and enzymatic activity of DprE1.(A) Structures of antitubercular compound families that target DprE1. BTZ043 (MIC 1 ng/mL) is in late preclinical development (5). Reduced BTZ043 analogues BTZ045 (amino) and BTZ046 (hydroxylamino) show MIC values >500-fold higher than that of BTZ043. DNB1 represents the dinitrobenzamide family of inhibitors (11) (MIC 0.072 μg/mL or 0.02 μM). VI-9376, a benzoquinoxaline, was also reported to target DprE1 (12) (MIC 1 μg/mL or 2.9 μM). (B) Epimerization reaction on the 2′ hydroxyl group of DPR, catalyzed by the mycobacterial DprE1/DprE2. DPR is converted into DPA, an essential precursor for the synthesis of the arabinan moiety of the mycobacterial cell wall (9). DprE1 catalyzes the first step through a FAD-dependent process that requires an electron acceptor for enzyme turnover, which] in vitro can be either molecular oxygen, DCPIP or menaquinone (MQ), as described in this report. BTZ043 was suggested to be converted into a nitroso derivative by DprE1 reduced flavin cofactor (13).