Abstract
To obtain microorganisms for the microbial conversion of ginsenosides in red ginseng extract (RGE), mushroom mycelia were used for the fermentation of RGE. After fermentation, total sugar contents and polyohenol contents of the RGEs fermented with various mushrooms were not a significant increase between RGE and the ferments. But uronic acid content was relatively higher in the fermented RGEs cultured with Lentus edodes (2155.6 μg/mL), Phelllinus linteus (1690.9 μg/mL) and Inonotus obliquus 26137 and 26147 (1549.5 and 1670.7 μg/mL) compared to the RGE (1307.1 μg/mL). The RGEs fermented by Ph. linteus, Cordyceps militaris, and Grifola frondosa showed particularly high levels of total ginsenosides (20018.1, 17501.6, and 16267.0 μg/mL, respectively). The ferments with C. militaris (6974.2 μg/mL), Ph. linteus (9109.2 μg/mL), and G. frondosa (7023.0 μg/mL) also showed high levels of metabolites (sum of compound K, Rh1, Rg5, Rk1, Rg3, and Rg2) compared to RGE (3615.9 μg/mL). Among four different RGE concentrations examined, a 20 brix concentration of RGE was favorable for the fermentation of Ph. linteus. Maximum biotransformation of ginsneoside metabolites (9395.5 μg/mL) was obtained after 5 days fermentation with Ph. linteus. Maximum mycelial growth of 2.6 mg/mL was achieved at 9 days, in which growth was not significantly different during 5 to 9 days fermentation. During fermentation of RGE by Ph. linteus in a 7 L fermenter, Rg3, Rg5, and Rk1 contents showed maximum concentrations after 5 days similar to flask fermentation. These results confirm that fermentation with Ph. linteus is very useful for preparing minor ginsenoside metabolites while being safe for foods.
Keywords: Red ginseng extract, Fermented red ginseng, Ginsenoside metabolites, Mushroom mycelia, Phelllinus linteus
INTRODUCTION
Ginseng (Panax ginseng of the Araliaceae family) is one of the most valuable oriental herbs. Typically, the dried root of the plant has been used as a healing drug and health tonic in countries such as China, Japan, and Korea since ancient times [1]. There are two different forms of ginseng: red ginseng is dried after steaming and is the most common form in traditional Korean medicine, while white ginseng is produced by sun drying. More than 40 ginsenosides have been isolated and identified in Asian ginseng. Various biological activities of ginsenosides have been reported, such as antisenescence, immuno-modulatory, antitumor, antiinflammatory protein anabolic, anti-diabetic, etc. [2-5].
Usually ginseng is administered orally, after which the ingredients are exposed to gastric juices and digestive and bacterial enzymes in the gastrointestinal tract. The characterization of the metabolism of ginseng saponins is important for explaining the pharmacological actions of ginseng [6-8]. Indeed, ginsenosides themselves have exerted various pharmacological activities, by directly being added to cell cultures in vitro or by being intraperitoneally or intravenously injected into experimental animals. These results have led to the misunderstanding that intact ginsenosides might be the real active principle in the body. However, Kobashi et al. [9] and Kobashi et al. [10] proposed the concept that plant glycosides act as a prodrug that is metabolized to the active form by intestinal bacterial deglycosylation. We revealed that the anticancer activities of ginsenosides after oral administration are based on their metabolites formed by intestinal bacterial deglycosylation [11,12] and fatty acid esterification [13,14].
To improve the oral absorption and bioavailability of these compounds, many different strategies have been used. Several studies have shown that the transformation of ginsenosides into deglycosylated ginsenosides (metabolites) is required in order for them to provide more effective in vivo physiological action [15]. Various transformation methods, including mild acid hydrolysis [16], enzymatic conversion [17], and microbial conversion [18], have been used. Chemical methods, however, produce side reactions such as epimerization, hydration, and hydroxylation, and most of the microorganisms used for the transformation of ginsenosides are not of food-grade standards.
In seeking to utilize the beneficial properties of ginsenoside metabolites using food-compatible microorganisms, we screened edible mushroom species capable of metabolizing ginsenosides from ginseng, and investigated changes in levels of total sugars, uronic acid, polyphenols, and ginsenoside metabolites during fermentation.
MATERIALS AND METHODS
Materials
Korean red ginseng extract (60 brix, RGE) was purchased at a ginseng market in Geumsan, Korea. Standard ginsenoside materials including compound K (CK), Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rg5, Rh1, Rh2, and Rk1 were purchased from Ambo Institute (Seoul, Korea). All other chemicals were of reagent grade and obtained from local suppliers.
Preculture and culture of mycelium of various mushrooms
The strains of Cordyceps sisnensis, Cordyceps militaris, Phelllinus linteus, Tremella fuciformis, Inonotus obliquus 26136, I. obliquus, Grifola frondosa, and Lentus edodes were received from Chungju University, and maintained on potato dextrose agar slants. The slants were inoculated and incubated at 25℃ for 7 days, and then stored at 4℃ for about 2 wk.
For the preculture, 40 mL of medium (potato dextrose broth; Difco Laboratories, Detroit, MI, USA) with an initial pH of 5.5 was prepared in a 300 mL flask, and then 10 mL of mycelium suspension from a slant culture was inoculated, followed by 7 days of incubation at 25℃ on a rotary shaker (150 rpm). A 25 g portion of RGE was poured into a 500 mL flask, dissolved with 100 mL of distilled water, and sterilized at 121℃ for 15 min (RGE medium). The precultured broths were inoculated into the RGE medium at 4 mL and incubated at 25℃ for 7 days with mild shaking (150 rpm).
Effect of initial red ginseng extract concentration
To investigate the impact of the initial RGE concentration, RGE was used at levels of 8.3 g (5 brix), 16.7 g (10 brix), 25 g (15 brix), and 33.3 g (20 brix) per 100 mL. The medium was inoculated by transferring 4 mL of preculture broth (with ca. 300-350 mg DW of cells/L) to 100 mL of medium in a 500 mL flask and incubated at 25℃ for 7 days with shaking (150 rpm).
Scaleup fermentation
Scaleup fermentation was carried out in a 7 L fermenter (Fermentec, Cheongwon, Korea) with a 5 L working volume of medium containing 333.3 g of RGE per liter. The fermentations were conducted at 25℃, with an aeration rate of 1.0 vvm, agitation speed of 150 rpm, and pH of 5.5. The seed cultures were transferred to the fermentation medium and were cultivated for 9 days.
Ginsenosides analysis with HPLC
After fermentation, the broths were transferred to centrifuge tubes and centrifuged at 4000 rpm for 10 min. The supernatants were collected and applied to an SPE C18 cartridge for sample clean-up [19]. The levels of 15 major ginsenosides were analyzed using an HPLC-based technique developed by Kim et al. [20].
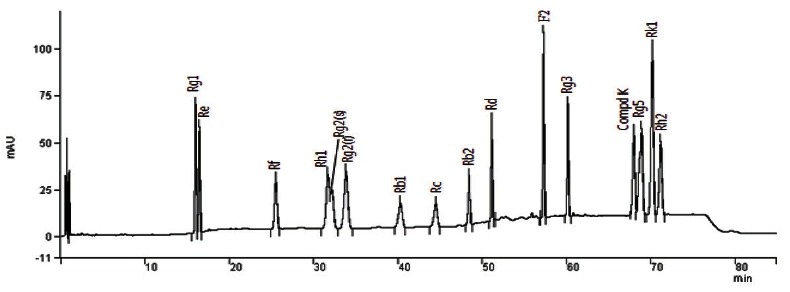
A Varian Prostar 200 HPLC system (Varian Inc., Palo Alto, CA, USA) equipped with a quaternary solvent delivery system, an autosampler, and a UV detector was used. The column configuration consisted of an IMtakt Cadenza CD-C18 (4.6×75 mm; Imtakt Corporation, Kyoto, Japan). UV absorption was measured at 203 nm. Gradient elution was employed using solvent A (10% acetonitrile) and solvent B (90% acetonitrile) at 40℃; the gradient program was as follows: 0→11 min, 11% B (isocratic); 11→15 min, 11→16% B; 15→16 min, 16 →20% B; 16→18 min, 20→21%; 18→24 min, 21% B (isocratic); 24→25 min, 21→22% B; 25→35 min, 22% B (isocratic); 35→36 min, 22→23% B; 36→40 min, 23% B (isocratic); 40→41 min, 23→24%; 41→45 min, 24% B (isocratic); 45→53 min, 24→37% B; 53→61 min, 37→45% B; 61→66 min, 45→46%; 66→73 min, 46→48% B; 73→75 min, 48% B (isocratic); 75→77 min, 48→11%; 77→85 min, 11% B (isocratic). The flow rate was kept at 1.3 mL/min and the sample injection volume was 5 μL. The level of total ginsenosides was determined by the sum of the 15 ginsenosides. Fig. 1 shows the HPLC chromatograms of the 15 standard ginsenosides.
Fig. 1. Chromatogram of standard ginsenosides by HPLC assay. An IMtakt Cadenza CD-C18 (4.6×75 mm) column was used. UV absorption was measured at 203 nm. Gradient elution was employed using solvent A (10% acetonitrile) and solvent B (90% acetonitrile) at 40℃.
Analytical methods
Total polyphenol content was determined using the Folin Ciocalteu method [21] adapted to a microscale using gallic acid as a standard (50-800 μg/L). Total sugar and uronic acid levels were determined using the phenol-sulfuric acid [22] and m-hydroxydiphenyl methods [23], respectively, using glucose and galacturonic acid as the respective standards. In all cases, the analyses were performed in triplicate unless otherwise specified. The values were averaged and standard deviations were calculated. All data were analyzed by one-way analysis of variance and Duncan’s multiple range tests using SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). The results were considered significant at p<0.05.
RESULTS
Total sugar, uronic acid, and polyphenol contents in fermented red ginseng extracts
The total sugar, uronic acid, and polyohenol contents of the RGEs fermented with various mushrooms are presented in Table 1. The total sugar contents of the fermented RGEs ranged from 319.6 to 458.4 mg/mL and the total sugar content of RGE (non-fermented RGE) was 444.8 mg/mL. The submerged culture ferments of mushrooms, except that with Ph. Linteus, showed lower levels of total sugars than RGE. The ferment cultured with C. militaris showed the lowest level of total sugars (319.6 mg/mL) among the ferments.
Table 1.
Total sugar, uronic acid, and polyphenol contents after 7 days fermentation of red ginseng extracts by mushroom mycelia
| Strain | Total sugar (mg/mL) | Uronic acid (μg/mL) | Polyphenols (μg/mL) |
|---|---|---|---|
|
| |||
| Red ginseng extract | 444.8±37.9ab | 1307.1±98.6cd | 1052.8±23.7a |
| Cordyceps sinensis | 393.1±29.0cd | 1276.8±356.1d | 1009.1±16.6b |
| Cordyceps militaris | 319.6±24.5e | 973.7±38.1e | 1058.8±17.4a |
| Phelllinus linteus | 458.4±8.6a | 1690.9±181.8b | 998.8±10.7b |
| Tremella fuciformis | 345.3±26.1de | 918.2±121.2e | 999.2±12.0b |
| Inonotus obliquus 26136 | 351.2±32.9de | 1549.5±53.2bc | 997.4±26.3b |
| Inonotus obliquus 26147 | 386.2±7.9cd | 1670.7±38.1b | 989.0±16.0b |
| Grifola frondosa | 361.0±22.3cde | 1115.2±90.9de | 1012.2±25.7b |
| Lentus edodes | 405.7±27.0bc | 2155.6±68.3a | 995.75±13.2b |
Uronic acid (an acidic polysaccharide) content was relatively higher in the fermented RGEs cultured with L. edodes (2155.6 μg/mL), Ph. linteus (1690.9 μg/mL), I. obliquus 26147 (1670.7 μg/mL), and I. obliquus 26136 (1549.5 μg/mL) compared to the RGE (1307.1 μg/mL). In particular, the RGE fermented with L. edodes had the highest level of uronic acid (p<0.05).
Also, for the other strains except C. militaris (1058.8 μg/g), polyphenol content (989.0-1012.2 μg/g) was lower in the fermented RGEs compared to the control (1052.8 μg/g, p<0.05).
Changes of ginsenoside composition in fermented red ginseng extracts
The ginsenoside compositions of the RGEs fermented by mushroom mycelia are shown in Table 2. The ginsenoside content of RGE was 13786.5 μg/mL and the total ginsenoside contents of the fermented RGEs were in a range of 11431.9-20018.1 μg/mL. The RGEs fermented by Ph. linteus, C. militaris, and G. frondosa showed particularly high levels of total ginsenosides (20018.1, 17501.6, and 16267.0 μg/mL, respectively). The ginsenosides Rg1 and Rb1 in the ferments with Ph. linteus (3230.3 μg/mL) and C. militaris (2981.4 μg/mL) also showed high levels compared to the RGE (2774.1 μg/mL).
Table 2.
Total gisenoside and ginsenoside metabolite contents after 7 days fermentation of red ginseng extracts (RGEs) by mushroom mycelia
| Gisenoside | Fermented RGE (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| RGE | Cordyceps sinensis | Cordyceps militaris | Phelllinus linteus | Tremella fuciformis | Inonotus obliquus 26136 | Inonotus obliquus 26147 | Grifola frondosa | Lentus edodes | ||
|
| ||||||||||
| Rg1 | 668.7±23.1 | 424.6±23.2 | 773.3±57.6 | 810.4±40.3 | 515.5±24.5 | 552.1±35.5 | 826.5±65.3 | 465.3±35.4 | 373.3±13.3 | |
| Re | 1974.9±48.5 | 1348.8±83.4 | 1688.6±76.4 | 1449.1±34.1 | 1271.2±76.2 | 1312.4±67.4 | 1380.0±70.5 | 1215.8±82.1 | 1170.9±67.9 | |
| Rf | 464.6±54.3 | 435.1±32.3 | 518.5±43.5 | 508.8±36.5 | 418.8±18.4 | 442.0±24.8 | 455.3±35.4 | 457.3±37.5 | 412.2±22.1 | |
| Rh1+Rg2(S) | 276.4±34.5 | 360.0±24.3 | 404.5±42.3 | 597.1±33.3 | 338.7±28.7 | 357.0±40.2 | 522.6±26.4 | 403.0±30.0 | 410.8±28.5 | |
| Rg2(R) | 67.1±8.9 | 123.7±10.3 | 139.0±10.7 | 229.5±18.7 | 123.0±10.3 | 131.2±13.5 | 165.1±10.2 | 147.0±10.7 | 125.7±7.8 | |
| Rb1 | 2105.4±203.4 | 1802.9±85.6 | 2208.0±105.6 | 2419.6±100.3 | 1365.1±56.8 | 1421.6±58.4 | 1627.0±67.4 | 2096.0±94.4 | 1324.5±44.5 | |
| Rc | 2224.3±142.3 | 1867.5±105.3 | 2386.2±165.4 | 2572.4±89.5 | 1412.9±40.6 | 1468.1±68.4 | 1705.6±30.5 | 2194.0±94.1 | 1391.9±31.9 | |
| Rb2 | 1674.2±45.2 | 1467.7±67.4 | 1841.4±50.5 | 2030.5±70.1 | 1122.8±43.3 | 1161.7±38.5 | 1352.5±32.5 | 1708.1±37.5 | 1100.8±40.8 | |
| Rd | 1058.7±87.5 | 777.7±65.3 | 1109.3±37.5 | 1117.7±45.4 | 550.3±22.2 | 580.5±20.1 | 670.3±30.5 | 1107.2±47.3 | 532.2±32.3 | |
| Rg3 | 459.6±25.3 | 844.3±34.8 | 1032.1±23.4 | 1188.1±23.1 | 740.8±18.9 | 788.0±33.5 | 846.6±25.4 | 1035.1±13.2 | 754.1±21.2 | |
| Rg5 | 1523.5±34.2 | 2649.5±68.9 | 3141.7±94.2 | 3874.0±100.2 | 2156.5±80.4 | 2349.1±84.5 | 2712.7±57.5 | 3184.0±81.3 | 2347.0±74.3 | |
| Rk1 | 1289.3±85.4 | 1850.8±79.8 | 2257.0±104.3 | 3220.9±59.5 | 1416.4±46.4 | 1554.5±78.4 | 2158.8±46.5 | 52253.8±83.1 | 1710.6±40.6 | |
| CK | 0.0 | 108.3±13.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Rh2 | 0.0 | 0.0 | 1.6±0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Total | 13786.5±792.6 | 14060.6±694.1 | 17501.6±811.9 | 20018.1±650.9 | 11431.9±466.7 | 12118.5±553.2 | 14423.1±498.1 | 16267.0±646.9 | 11654.0±425.2 | |
| Rg1+Rb1 | 2774.1±226.5 | 2227.5±108.8 | 2981.4±163.2 | 3230.3±140.6 | 880.5±81.3 | 1973.8±93.9 | 2453.5±132.7 | 2561.4±129.8 | 1697.8±57.8 | |
| Metabolites1) | 3615.9±228.3 | 5936.6±231.6 | 6974.2±274.9 | 9109.2±234.8 | 4775.6±184.7 | 5179.8±250.1 | 6406.0±166.0 | 7023.0±218.3 | 5348.3±172.4 | |
CK, compound K.
1)Sum of CK, Rh1, Rg5, Rk1, Rg3, and Rg2.
In recent decades, many studies have focused on the pharmaceutical activities of minor ginsenosides such as ginsenosides Rd, Rg3, Rh2, and ginsenoside K (CK), as their activities are found to be superior to those of major ginsenosides. These minor ginsenosides are present in ginseng only in small percentages and are known to be produced by the hydrolysis of sugar moieties of major ginsenosides. In addition, ginseng and its derived products are orally administered in most cases, and a number of metabolites are produced via the degradation of ginsenosides by acid or intestinal bacteria in the gastrointestinal tract. Ginsenoside metabolites (sum of CK, Rh1, Rg5, Rk1, Rg3, and Rg2) were found in the RGE fermented using mushroom mycelia (Table 2). In particular, the ferments with Ph. linteus (9109.2 μg/mL), G. frondosa (7023.0 μg/mL), and C. militaris (6974.2 μg/mL), showed high levels of metabolites compared to the RGE (3615.9 μg/mL).
Ginseng is usually administered orally, after which its components are exposed to gastric juices and digestive and bacterial enzymes in the gastrointestinal tract. The intestinal bacteria population is variable, depending on the conditions of the host, including diet, health, and even stress. To overcome such variations, a microorganism that can produce ginsenoside metabolites would be deemed valuable. Therefore, the Ph. linteus strain was selected for the production of ginsenoside metabolites.
Changes of ginsenosides in RGE fermented with Phelllinus linteus under various RGE concentrations
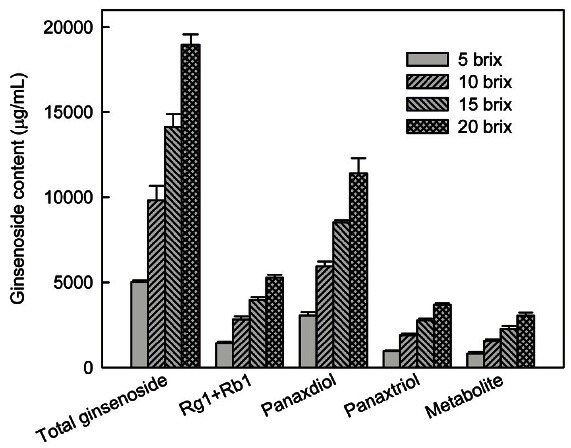
The osmotic pressure caused by a high RGE concentration may be detrimental to fermentation. To find a suitable RGE concentration for ginsenoside transformation by Ph. linteus, RGE was added at different concentrations ranging from 8.3 g (5 brix) to 33.3 g (20 brix) per 100 mL. After 7 days fermentation, the ginsenoside contents (total ginsenosides, Rg1 and Rb1, protopanaxadiols, protopanaxatriols, and metabolites) in the ferment increased with an increase in concentration (Fig. 2). Ginsenoside content showed a similar fold increase with the fold increase in RGE concentration until 20 brix. However, mycelial growth was inhibited at the above 20 brix because of decrease of mycelial dry weight at the concentration (data not shown). Therefore, the 20 brix concentration of RGE was favorable for the fermentation of Ph. linteus. Using a high concentration of RGE may have some advantages such as decreased production time and cost, and easier control of procedures.
Fig. 2. Ginsenoside contents after 7 days fermentation with Phelllinus linteus under various red ginseng extract (RGE) concentrations. RGE was used at levels of 8.3 g (5 brix), 16.7 g (10 brix), 25 g (15 brix), and 33.3 g (20 brix) per 100 mL. The medium was inoculated by transferring 4 mL of preculture broth to 100 mL of medium in a 500 mL flask and incubated at 25℃ for 7 days with shaking (150 rpm).
Mycelial growth, total gisenosides, and metabolite changes in a submerged culture of Phelllinus linteus in a 7 L fermenter
Fig. 3 shows the typical time course of mycelial growth and ginsenoside changes in the 7 L fermenter. Maximum mycelial growth of 2.6 mg/mL was achieved at 9 days, in which growth was not significantly different during 5 to 9 days of fermentation. The total ginsenoside and metabolite contents of the RGE fermented by Ph. linteus increased with increasing fermentation time until 5 days.
Fig. 3. Time profiles of mycelia growth, total ginsenoside, and their metabolite changes in submerged culture of Phelllinus linteus in a 7 L fermenter. Fermentations were conducted at 25℃, an aeration rate of 1.0 vvm, agitation speed of 150 rpm, and pH of 5.5. The seed cultures were transferred to fermentation medium and were cultivated for 9 days.
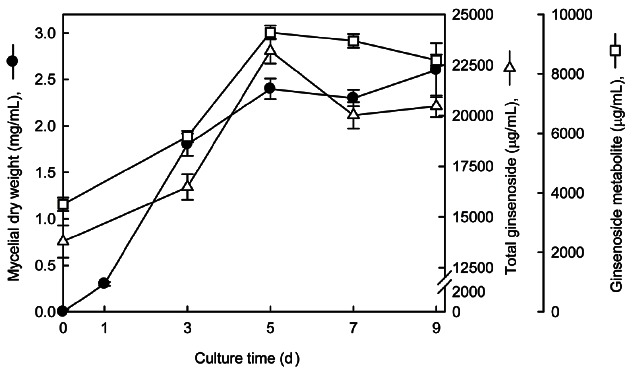
Table 3 shows changes in ginsensides during fermentation of RGE by Ph. linteus. Rg1 and Rb1 content increased with increasing fermentation time. In particular, both Rb1 and Rg1 increased with increasing fermentation time (Table 3). Rg3 and Rk1 contents showed maximum concentrations after 5 days, similar to flask fermentation. A comparable result was observed in our previous flask culture (Table 3). After 5-days fermentation, contents were 140.1% to 261.1% higher than those of non-fermented RGE.
Table 3.
Total gisenoside and ginsenoside metabolite contents in fermentations of red ginseng extracts by Phelllinus linteus
| Ginsenoside (μg/mL) | Culture time (d) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 3 | 5 | 7 | 9 | |
|
| |||||
| Rg1 | 668.7±23.1 | 698.9±48.2 | 703.5±35.7 | 810.4±40.3 | 819.3±39.8 |
| Re | 1974.9±48.5 | 1576.6±66.7 | 1660.8±60.8 | 1449.1±34.1 | 1705.0±57.0 |
| Rf | 464.6±54.3 | 456.7±26.7 | 508.9±29.8 | 508.8±36.5 | 491.8±28.5 |
| Rh1+Rg2(S) | 276.4±34.5 | 506.2±44.6 | 696.5±55.6 | 597.1±33.3 | 675.7±47.5 |
| Rg2(R) | 67.1±8.9 | 192.4±12.4 | 217.5±17.5 | 229.5±18.7 | 183.7±13.7 |
| Rb1 | 2105.4±203.4 | 2130.1±90.1 | 2542.3±85.4 | 2419.6±100.3 | 2640.3±64.2 |
| Rc | 2224.3±142.3 | 2197.0±67.5 | 3515.6±65.4 | 2572.4±89.5 | 2077.3±77.3 |
| Rb2 | 1674.2±45.2 | 2108.6±68.2 | 2381.1±51.8 | 2030.5±70.1 | 2142.3±42.3 |
| Rd | 1058.7±87.5 | 2149.8±49.7 | 2397.6±39.7 | 1117.7±45.4 | 2327.7±27.7 |
| Rg3 | 459.6±25.3 | 1205.7±20.5 | 1434.8±34.8 | 1188.1±23.1 | 954.0±25.4 |
| Rg5 | 1523.5±34.2 | 2456.8±68.5 | 3268.9±62.3 | 3874.0±100.2 | 3345.8±54.8 |
| Rk1 | 1289.3±85.4 | 1530.8±60.8 | 3778.0±78.4 | 3220.9±59.5 | 3305.8±58.4 |
| CK | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rh2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 13786.5±792.6 | 16480.0±623.9 | 23197.4±617.2 | 20018.1±650.9 | 20466.9±536.6 |
| Protopanaxdiols1) | 7522.2±503.7 | 9791.2±296.0 | 12271.4±277.1 | 9328.3±328.4 | 10141.6±236.9 |
| Protopanaxtriols2) | 3451.7±169.3 | 3430.8±198.6 | 3787.2±199.4 | 3594.9±162.9 | 3875.5±186.5 |
| Metabolite3) | 3615.9±228.3 | 5891.9±194.4 | 9395.5±231.1 | 9109.2±234.8 | 8465.0±186.1 |
1)Sum of Rb1, Rb2, Rc, Rd, Rg3, Rh2 and CK.
2)Sum of Re, Rf, Rg1, Rg2 and Rh1.
3)Sum of CK, Rh1, Rg5, Rk1, Rg3, and Rg2.
The combined content of ginsenosides Rb1, Rb2, Rc, Rd, Rg3, CK, and Rh2, which are major ginsenosides (protopanaxadiol type), only changed from 7522.2 μg/mL to 9791.2 to 12271.4 μg/mL during fermentation. On the other hand, the content of protopanaxtriols (Rg1, Re, Rf, Rh1, and Rg2) was slightly change from 3451.7 μg/mL to 3430.8-3875.5 μg/mL throughout fermentation.
DISCUSSION
Ginsenosides, glycosides with steroids or triterpenes as aglycones, are an important class of physiologically active compounds occurring in many herbs. In recent years, the sugar chains of saponins have been found to be closely related to the biological activity of ginsenosides, and modification of the sugar chains may markedly change the biological activity of ginsenosides [24,25]. Recently, it was suggested that orally ingested ginsenosides are metabolized by human intestinal bacteria, and deglycosylated ginsenoside metabolites are known to be more readily absorbed into the bloodstream and act as active compounds [15,17]. Lee et al. [26] also suggested that the efficiency of the conversion and transformation pathways differs greatly owing to the diversity of resident microflora between individuals. Metabolites are generally prepared via the biotransformation of ginsenosides in the presence of human intestinal bacteria [6,18], soil fungi [27], or certain commercial enzymes [28,29]. Therefore, ginsenosides with more uniform and targeted biological functions may be attained by using specially isolated microorganisms. In this study, we screened for available microorganisms using RGE as a substrate and produced fermented RGE using Ph. linteus.
For the utilization of Korean ginseng (Panax ginseng) in a functional drink, Park et al. [30] prepared fermented Korean ginseng with mushroom mycelia (Ganoderma lucidum, Hericium erinaceum, and Ph. linteus) by solid culture. The Korean ginseng fermented with mushroom mycelia by solid culture contained chemical ingredients different from Korean ginseng, and it might provide beneficial immunostimulating activity. Through two screening steps, a strain of fungus (ECU2042) capable of selectively transforming ginsenoside Rg3 into ginsenoside Rh2 was isolated from soil samples and identified as Fusarium proliferatum. In the preparation of ginsenoside Rh2 using the crude cell-free extract of F. proliferatum ECU2042, a higher conversion of 60.3% was obtained as compared to that previously reported. Therefore, this method was considered to be potentially useful for the practical preparation of ginsenoside Rh2 [31].
The fermented RGE had increased total ginsenoside and metabolite contents, especially for Rh1, Rh2, Rg5, Rk1, Rg2, and Rg3 (Table 2). Red ginseng mainly contains ginsenosides such as Rg3, Rb1, Rb2, and Rc. Rh1, Rh2, and Rg3, which are representative constituents in red ginseng, and are produced from protopanaxadiol ginsenosides by steaming raw ginseng. Table 3 shows the changes in ginsensides during fermentation of RGE by Ph. linteus. Rg3, Rg5, and Rk1 contents showed maximum concentrations after 5 days, similar to flask fermentation.
Through several studies, investigators have reported that the ginsenosides Rb1, Rb2, and Rc are metabolized to CK, and that this metabolite induces antimetastatic or anticarcinogenic activity. It has also been reported that a deglycosylation process is required for ginsenosides to exhibit their greatest effects in vivo, and that their clinical efficacy varies with the hydrolyzing potential of the components of the intestinal microflora. Ginsenosides may be degraded by a variety of methods, including mild acid hydrolysis, enzymatic activity, or microbial activity [17]. However, each of these methods has demonstrated some defects when used routinely. Many kinds of bacteria have been used in an attempt to overcome these obstacles, including Prevotella oris, Eubacterium sp. A-44, Bifidobacterium sp. K506, Bacteroides sp. JY6, and Fusobacterium sp. K-60, all of which can cooperatively metabolize ginsenosides [18,32].
Ginsenoside Rh1 is produced from Re via Rg1 and Rg2 by Bifidobacterium sp. Int57, Bifidobacterium Sp. SJ32, Aspergillus niger, and Aspergillus usamii [33]. Rg2 has been found to reduce the acetylcholine-evoked secretion of catecholamines from cultured bovine adrenal chromaffin cells [34]. Rh1 is known to possess anti-allergic and anti-inflammatory activity [35]. Therefore, fermentation may be a means to simultaneously obtain red ginseng components that have immunopotentiating activity and potent cytotoxicity against tumor cells, and that are easily absorbed in the human intestinal tract.
We attempted to identify a suitable mushroom mycelium that could replace the need for human intestinal bacteria in the biotransformation of ginsenosides into their metabolites. For the production of fermented RGE, this study used Ph. linteus, whose enzymes are increasingly being used as an effective means of structure modification, as well as for the metabolism study of natural and synthetic organic compounds. The RGE underwent many changes during fermentation in terms of total sugar, uronic acid, and ginsenoside composition, and had increases in absorbable ginsenosides such as Rh1, Rg3, Rg5, and Rk1.
Collectively, our results indicate that Ph. linteus is a very useful tool in the structure modification and metabolism study of ginseng, as well as for the preparation of minor ginsenosides and intestinal bacterial metabolites from ginseng extract, which possess both selectivity and efficiency.
Acknowledgments
This work was supported by a research grant from the Academic Research Foundation of Hankyong National University for a scholarly exchange program in 2010.
References
- 1.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dey L, Zhang L, Yuan CS. Anti-diabetic and anti-obese effects of ginseng berry extract: comparison between intraperitoneal and oral administrations. Am J Chin Med. 2002;30:645–647. doi: 10.1142/S0192415X02000648. [DOI] [PubMed] [Google Scholar]
- 3.He W, Liu G, Chen X, Lu J, Abe H, Huang K, Manabe M, Kodama H. Inhibitory effects of ginsenosides from the root of Panax ginseng on stimulus-induced superoxide generation, tyrosyl or serine/ threonine phosphorylation, and translocation of cytosolic compounds to plasma membrane in human neutrophils. J Agric Food Chem. 2008;56:1921–1927. doi: 10.1021/jf073364k. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Sung JH, Lee SJ, Moon CK, Lee BH. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999;144:39–43. doi: 10.1016/S0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, Chae IG, Lee SG, Jeong HJ, Lee EJ, Lee EJ, Lee IS. Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin-induced diabetic rats. J Ginseng Res. 2010;34:104–112. doi: 10.5142/jgr.2010.34.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa H, Sung JH, Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 7.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 8.Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 9.Kobashi K, Akao T, Hattori M, Namba T. Metabolism of drugs by intestinal bacteria. Bifidobact Microflora. 1992;11:9–23. [Google Scholar]
- 10.Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Bifidobact Microflora. 1997;16:1–7. [Google Scholar]
- 11.Wakabayashi C, Hasegawa H, Murata J, Saiki I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res. 1997;9:411–417. [PubMed] [Google Scholar]
- 12.Wakabayashi C, Hasegawa H, Murata J, Saiki I. The expression of in vivo anti-metastatic effect of ginseng protopanaxatriol saponins is mediated by their intestinal bacterial metabolites after oral administration. J Trad Med. 1997;14:180–185. [Google Scholar]
- 13.Hasegawa H, Lee KS, Nagaoka T, Tezuka Y, Uchiyama M, Kadota S, Saiki I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol Pharm Bull. 2000;23:298–304. doi: 10.1248/bpb.23.298. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H, Saiki I. Oleoyl triterpene glycoside biosynthesized from ginseng suppresses growth and metastasis of murine melanoma B16-F10 tumor via immunostimulation. J Trad Med. 2000;17:186–193. [Google Scholar]
- 15.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 16.Han BH, Park MH, Han YN, Woo LK, Sankawa U, Yahara S, Tanaka O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982;44:146–149. doi: 10.1055/s-2007-971425. [DOI] [PubMed] [Google Scholar]
- 17.Ko SR, Choi KJ, Uchida K, Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1 , and F1 from protopanaxatriol-type ginseng saponin mixture. Planta Med. 2003;69:285–286. doi: 10.1055/s-2003-38476. [DOI] [PubMed] [Google Scholar]
- 18.Bae EA, Han MJ, Choo MK, Park SY, Kim DH. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 19.Lou DW, Saito Y, Jinno K. Solid-phase extraction and high-performance liquid chromatography for simultaneous determination of important bioactive ginsenosides in pharmaceutical preparations. Chromatographia. 2005;62:349–354. doi: 10.1365/s10337-005-0640-6. [DOI] [Google Scholar]
- 20.Kim SJ, Murthy HN, Hahn EJ, Lee HL, Paek KY. Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng C. A. Meyer). Sep Purif Technol. 2007;56:401–406. doi: 10.1016/j.seppur.2007.06.014. [DOI] [Google Scholar]
- 21.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 22.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 23.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 24.Shibata S, Ando T, Tanaka O. Chemical studies on the oriental plant drugs. XVII. The prosapogenin of the ginseng saponins (ginsenosides-Rb1, -Rb2, and -Rc). Chem Pharm Bull (Tokyo) 1966;14:1157–1161. doi: 10.1248/cpb.14.1157. [DOI] [PubMed] [Google Scholar]
- 25.Kim M, Ko S, Choi K, Kim S. Distribution of saponin in various sections of Panax ginseng root and changes of its contents according to root age. Korean J Ginseng Sci. 1987;11:10–16. [Google Scholar]
- 26.Lee DS, Kim YS, Ko CN, Cho KH, Bae HS, Lee KS, Kim JJ, Park EK, Kim DH. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- 27.Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y. Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J Agric Food Chem. 2007;55:9373–9379. doi: 10.1021/jf070354a. [DOI] [PubMed] [Google Scholar]
- 28.Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, Lee JK, Baik MY, Park CS, Ahn SC. Biotransformation of Korean Panax ginseng by Pectinex. Biol Pharm Bull. 2006;29:2472–2478. doi: 10.1248/bpb.29.2472. [DOI] [PubMed] [Google Scholar]
- 29.Chang KH, Jee HS, Lee NK, Park SH, Lee NW, Paik HD. Optimization of the enzymatic production of 20(S)-ginsenoside Rg(3) from white ginseng extract using response surface methodology. N Biotechnol. 2009;26:181–186. doi: 10.1016/j.nbt.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Park CK, Kim H, Tu Q, Yu KW, Jeong HS, Lee HY, Jeong JH. Chemical composition and immunostimulating activity of the fermented Korean ginseng (Panax ginseng C. A. Meyer) with mushroom mycelium by solid culture. J Korean Soc Food Sci Nutr. 2009;38:1145–1152. doi: 10.3746/jkfn.2009.38.9.1145. [DOI] [Google Scholar]
- 31.Su JH, Xu JH, Lu WY, Lin GQ. Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolated Fusarium proliferatum ECU2042. J Mol Catal B Enzym. 2006;38:113–118. doi: 10.1016/j.molcatb.2005.12.004. [DOI] [Google Scholar]
- 32.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.FMJ04001X4. [DOI] [PubMed] [Google Scholar]
- 33.Lee BH, You HJ, Park MS, Kwon B, Ji GE. Transformation of the glycosides from food materials by probiotics and food microorganisms. J Microbiol Biotechnol. 2006;16:497–504. [Google Scholar]
- 34.Tachikawa E, Kudo K, Hasegawa H, Kashimoto T, Sasaki K, Miyazaki M, Taira H, Lindstrom JM. In vitro inhibition of adrenal catecholamine secretion by steroidal metabolites of ginseng saponins. Biochem Pharmacol. 2003;66:2213–2221. doi: 10.1016/j.bcp.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]


