Table 4.
Other consent recommendations
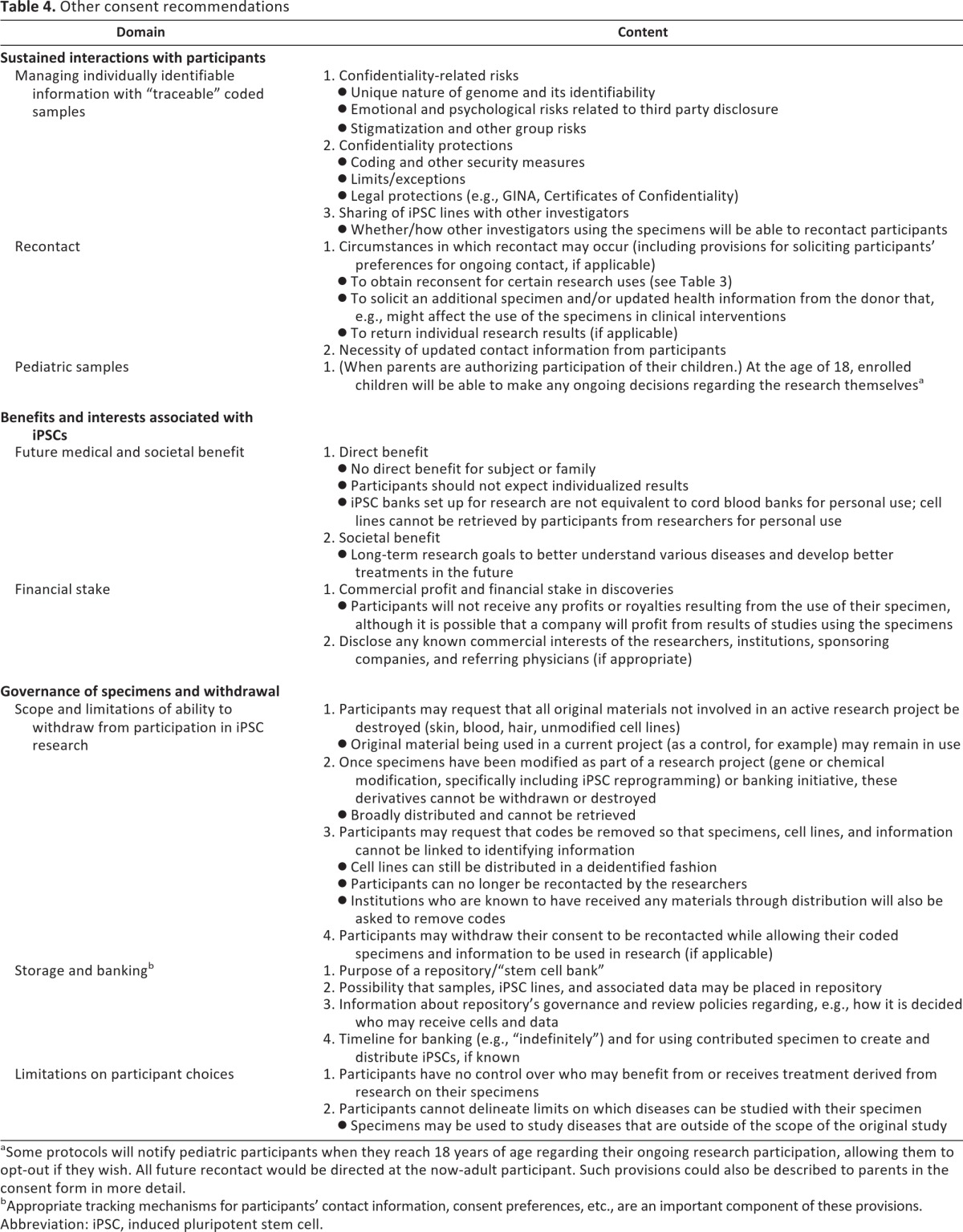
Some protocols will notify pediatric participants when they reach 18 years of age regarding their ongoing research participation, allowing them to opt-out if they wish. All future recontact would be directed at the now-adult participant. Such provisions could also be described to parents in the consent form in more detail.
Appropriate tracking mechanisms for participants' contact information, consent preferences, etc., are an important component of these provisions.
Abbreviation: iPSC, induced pluripotent stem cell.
