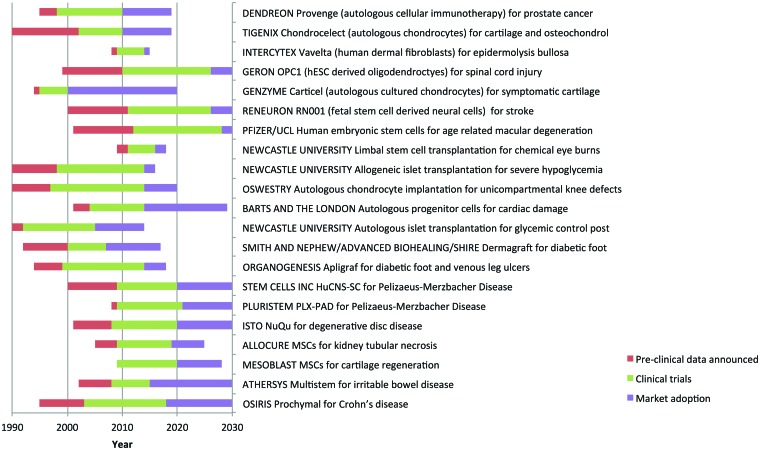
Figure 4.
Time lines of the development of the cellular therapies illustrated in Figure 3, with predictions beyond 2010. Three phases are shown: preclinical development (red), the clinical trials phase (green), and the time to market adoption (purple). Events before the end of 2010 are taken from information in the public domain. We have made the following assumptions in arriving at our estimates beyond 2010: (a) the cell therapy is safe and effective in clinical trials; (b) adoption will be more rapid and for longer periods of time for orphan indications as there is no existing treatment, they are fast tracked by the regulators, and they have extended intellectual property protection; (c) where not complete, clinical trials are estimated to take twice as long as those documented by the pharmaceutical industry [59]; (d) cellular therapies will first be taken through either the U.S. Food and Drug Administration or the European Medicines Agency, not both simultaneously, and the granting of a license (advanced therapeutic medicinal product or human cells, tissues, and cellular and tissue-based products) by one regulator will be followed 3 years later by the other; and (e) widespread adoption does not, in all cases, represent full market coverage; it is perhaps best defined as the market plateau. In estimating time to widespread adoption, we have considered the following factors: (a) market size; (b) clinical complexity; (c) substantial benefit relative to existing treatments; (d) competing cellular therapies; (e) supply; and (f) cost of goods. As an illustration, three worked examples are shown in Table 2. Abbreviations: hESC, human embryonic stem cell; MSC, mesenchymal stem cell.