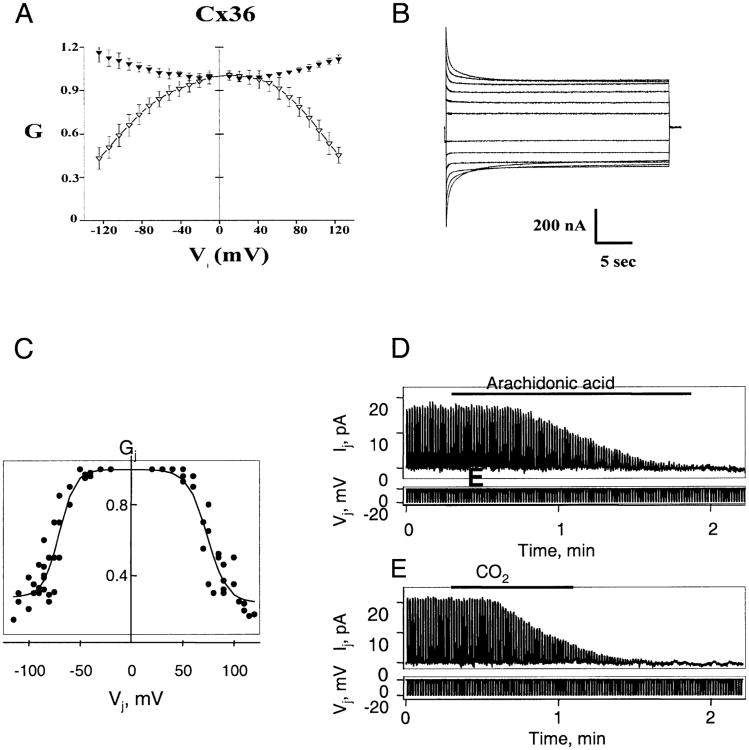
Fig. 6.
Voltage and chemical gating of Cx36 gap junctions. (A–C) Voltage dependence of Cx36 homotypic junctions expressed in Xenopus oocytes and transfected HeLa cells. (A) Graph of initial and steady-state Gj (filled and open symbols, respectively) as a function of Vj. Gj is gj normalized to its value at Vj = 0. Data represent mean values obtained from 9 Xenopus oocyte cell pairs with maximum gs less than 5 μS. Both initial and steady-state Gj- Vj relations are nearly symmetric about Vj = 0 with initial Gj showing an increase and steady-state Gj a decrease with increasing Vjs of either polarity. (B) Representative junctional currents for Vj steps up to ±120 mV in 20 mV increments. A +20 mV Vj step, 200 msec in duration, preceded each long-duration (30 sec) Vj step. Upward and downward currents are elicited by negative and positive Vj 's, respectively. Current calibration, 200 nA. Time calibration, 5 sec. (C) Graph of steady-state Gj as a function of Vj in HeLa-Cx36 cell pairs. Conductance is normalized as described in A. Solid lines are fits of the experimental data with the Boltzmann equation (see Results for details). (D and E) Examples of chemical gating of Cx36 homotypic junctions expressed in transfected HeLa cells. Junctional current, Ij, was measured by applying repeated negative pulses (Vj) of 20 mV to one cell of a pair. Holding potentials of −20 mV were maintained for both cells in each case. (D) Application of arachidonic acid (see horizontal bar) to a cell pair in which gj≈ 0.8 nS produced full uncoupling. (E) Application of 100% CO2 (horizontal bar) to a cell pair with gj ≈ 1 nS produced full uncoupling within ∼1 min.