This study demonstrates that podocalyxin, a heavily glycosylated type 1 transmembrane protein, is a glycoprotein ligand of rBC2LCN on human induced pluripotent stem (iPS) cells and embryonic stem (ES) cells. When analyzed by DNA microarray, podocalyxin was found to be highly expressed in both iPS cells and ES cells. The carbohydrate antigens of rBC2LCN are expressed on O-glycans of podocalyxin, since alkaline hydrolysis greatly reduced the binding of rBC2LCN to human iPS cells and ES cells. rBC2LCN bound to an O-glycan carrying H type 3 epitope structure isolated from iPS cells, suggesting that H type 3 is a novel pluripotency glycan marker.
Keywords: Differentiation antigens, Embryonic stem cells, Glycosaminoglycan, Induced pluripotent stem cells, Microarray, Reprogramming
Abstract
In comprehensive glycome analysis with a high-density lectin microarray, we have previously shown that the recombinant N-terminal domain of the lectin BC2L-C from Burkholderia cenocepacia (rBC2LCN) binds exclusively to undifferentiated human induced pluripotent stem (iPS) cells and embryonic stem (ES) cells but not to differentiated somatic cells. Here we demonstrate that podocalyxin, a heavily glycosylated type 1 transmembrane protein, is a glycoprotein ligand of rBC2LCN on human iPS cells and ES cells. When analyzed by DNA microarray, podocalyxin was found to be highly expressed in both iPS cells and ES cells. Western and lectin blotting revealed that rBC2LCN binds to podocalyxin with a high molecular weight of more than 240 kDa in undifferentiated iPS cells of six different origins and four ES cell lines, but no binding was observed in either differentiated mouse feeder cells or somatic cells. The specific binding of rBC2LCN to podocalyxin prepared from a large set of iPS cells (138 types) and ES cells (15 types) was also confirmed using a high-throughput antibody-overlay lectin microarray. Alkaline digestion greatly reduced the binding of rBC2LCN to podocalyxin, indicating that the major glycan ligands of rBC2LCN are presented on O-glycans. Furthermore, rBC2LCN was found to exhibit significant affinity to a branched O-glycan comprising an H type 3 structure (Ka, 2.5 × 104 M−1) prepared from human 201B7 iPS cells, indicating that H type 3 is a most probable potential pluripotency marker. We conclude that podocalyxin is a glycoprotein ligand of rBC2LCN on human iPS cells and ES cells.
Introduction
Human induced pluripotent stem (iPS) cells and embryonic stem (ES) cells with characteristics of self-renewal and pluripotency are attractive sources of cells for cell-replacement therapies. Before pluripotent stem cells can be used clinically, however, an important safety concern to be addressed is that residual undifferentiated cells could form tumors in patients. The use of cell surface pluripotency markers would serve as a standard and indeed a powerful technique to detect, and ideally remove, such teratoma-forming undifferentiated cells. Monoclonal antibodies that include anti-SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81 have been used as probes for this purpose [1–3]. Among them, SSEA-3 and SSEA-4 are carried by globo-series glycolipids, consisting of Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc-Cer and its α2–3-sialylated form (Siaα2–3Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc-Cer), respectively. On the other hand, TRA-1-60 and TRA-1-81 markers have been reported to be expressed on podocalyxin, a heavily glycosylated membrane protein [4]. Their carbohydrate epitopes were first reported to be keratan sulfate but were recently identified by glycan microarray analysis to be type 1 N-acetyllactosamine (Galβ1–3GlcNAc) [5]. More recently, anti-SSEA-5 was proposed as a novel antibody probe to detect as well as remove human iPS cells and ES cells through its specificity to H type 1 glycan (Fucα1–2Galβ1–3GlcNAc), although its carrier protein has not been identified [6]. It should be noted that all of these cell surface pluripotency markers are “carbohydrate antigens” that have been known to reflect the degree of cell differentiation/undifferentiation [3].
To understand the cellular glycome of human iPS cells and ES cells in a comprehensive way, we previously performed glycome analysis of a large set of human iPS cells (114 cell types) and ES cells (9 cell types) using an advanced high-density lectin microarray [7]. In that study, the expression of α2–6Sia, α1–2Fuc, and type 1 LacNAc was strongly indicated to be increased upon induction of pluripotency [7], and consistent with this, responsible glycosyltransferase genes, that is, ST6Gal1/2, FUT1/2, and B3GalT5, involved in the synthesis of these glycan epitopes showed significant increases in expression levels [7]. Furthermore, we found that a lectin designated rBC2LCN (recombinant N-terminal domain of BC2L-C), which was originally derived from Burkholderia cenocepacia, bound all of the undifferentiated cells tested, but not at all to any of the differentiated cells [7, 8]. Notably, this lectin was shown to have a strong preference to bind the defined epitope structure Fucα1–2Galβ1–3GlcNAc/GalNAc (H type 1/3/4), which comprises two of the above characteristics of pluripotency, that is, α1–2Fuc and type 1 LacNAc [7].
rBC2LCN is a small protein with a molecular weight of 15 kDa having a compact jellyroll architecture composed of 11 β strands and a short α helix, similar to the structure of tumor necrosis factor-like proteins [8]. rBC2LCN can be expressed at high levels in a soluble form in the cytoplasm of Escherichia coli (up to 80 mg/l) and can be purified to homogeneity in a one-step sugar-immobilized affinity chromatography approach [7]. rBC2LCN is highly specific to the defined glycan epitope Fucα1–2Galβ1–3GlcNAc/GalNAc, which is contained in glycans such as H type 1 (Fucα1–2Galβ1–3GlcNAc), H type 3 (Fucα1–2Galβ1–3GalNAc), Leb (Fucα1–2Galβ1–3(Fucα1–4)GlcNAc), and Globo H (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc) [8]. Therefore, rBC2LCN, unlike antibody, could serve as a novel type of detection reagent of pluripotent stem cells [9], particularly given that it is cost-effective and easy to produce in large amounts.
Here we demonstrate that podocalyxin, previously known as a Tra-1-60/81 carrier protein, functions as the glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2CLN on undifferentiated iPS cells and ES cells [10]. Furthermore, we report evidence that H type 3 is a most probable pluripotent glycan marker responsible for this lectin probe.
Materials and Methods
Cell
Human endometrium (UtE1104), amnion (AM936EP), and placental artery endothelium (PAE551) were collected by scraping tissues from surgical specimens as a therapy under signed informed consent, with ethical approval of the Institutional Review Board of the National Institute for Child Health and Development of Japan [11]. All experiments handling human cells and tissues were performed in line with the tenets of the Declaration of Helsinki. Endometrium, amnion, and placental artery endothelium cell lines were independently established. However, their actual origins remain to be clarified. Human iPS cells (MRC5-iPS, AM-iPS, UtE-iPS, and PAE-iPS) were then generated via procedures described by Yamanaka and colleagues [12, 13] and others [7, 14–16]. iPS201B7 and iPS253G1 human iPS cells were cultured in DMEM/Ham's F-12 medium (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 20% Knockout serum replacement (KSR) (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), minimal essential medium (MEM) nonessential amino acids (Invitrogen), and 10 ng/ml recombinant human basic fibroblast growth factor (Wako) on mitomycin C-treated mouse embryo fibroblast feeder cells. Human iPS cells (TIG/MKOS nos. 19, 56, and 106) were generated through reprogramming by Sendai virus infection-mediated expression of OCT4, SOX2, KLF4, and c-MYC as previously described [17]. The production of iPS cells was confirmed by immunofluorescence staining using established stem cell markers (Nanog, Oct3/4, SSEA4, and TRA-1-60) and analysis of the capacity for teratoma formation (supplemental online Fig. 1). Human ES cells (KhES1, KhES2, KhES3, and HUES1) were obtained and maintained as previously described [18, 19]. A list of the cell lines used in this study and their associated characteristics is given in supplemental online Table 1.
Immunoprecipitation of Human Podocalyxin
Hydrophilic fractions of cells were prepared using CelLytic MEM Protein Extraction (Sigma-Aldrich) in accordance with the manufacturer's procedures [14, 20]. Dynabeads M-280 streptavidin (Invitrogen; 10 μl) immobilized with biotinylated anti-podocalyxin polyclonal antibody (pAb) (1 μg) were incubated with hydrophilic fractions at 4°C overnight with agitation. After the beads were washed with 200 μl of phosphate-buffered saline (10 mM phosphate-buffered saline, pH 7.4, 140 mM NaCl, 2.7 mM KCl) containing 1% Triton X-100 (PBST) three times, bound podocalyxin was eluted with 50 μl of Tris-buffered saline (TBS) containing 0.2% SDS at 95°C for 10 minutes.
Western and Lectin Blotting
One microgram of the hydrophilic fractions or 5 μl of the immunoprecipitated samples described above were electrophoresed under reducing conditions on 5%–20% polyacrylamide gel (Dream Realization & Communication, Tokyo, Japan, http://www.drc2002.com/index.html; catalog no. NTH-676HP). The separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with BlockAce (Funakoshi, Tokyo, Japan, http://www.funakoshi.co.jp; catalog no. BUF029), the membrane was incubated with horseradish peroxidase (HRP)-conjugated rBC2LCN (1 μg/ml) or goat anti-podocalyxin pAb (0.2 mg/ml; R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com; catalog no. AF1658) followed by HRP-conjugated donkey anti-goat IgG (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com; catalog no. 705-035-003). Silver staining of the gel was also performed using a silver staining kit (Wako Chemical, Osaka, Japan, http://www.wako-chem.co.jp/english; catalog no. 299-58901). The HRP labeling of rBC2LCN was performed using a peroxidase labeling kit (Dojindo Molecular Technologies Inc., Gaithersburg, MD, http://www.dojindo.com; LK11). The immunoprecipitated podocalyxin (10 μl) was heat-denatured at 95°C for 5 minutes and treated with 1 μl of Peptide:N-glycosidase F (PNGase F; ProZyme, Hayward, CA, http://www.prozyme.com; catalog no. GKE-5003) at 37°C overnight to remove N-glycans. Selective β-elimination of O-glycans from podocalyxin was performed by incubating the Western blot with 0.05 M NaOH for 16 hours at 40°C before probing with HRP-labeled rBC2LCN as described [21, 22].
Production of High-Density Lectin Microarray
The high-density lectin microarray immobilizing 96 lectins including rBC2LCN was prepared as previously described [7, 23, 24]. Each lectin was spotted at 0.5 mg/ml in triplicate (supplemental online Fig. 2).
Antibody-Overlay Lectin Microarray
Immunoprecipitated samples were prepared from mouse embryonic fibroblasts (MEFs) (no. 1), somatic cells (nos. 2–15), iPS cells (nos. 16–153), and ES cells (nos. 154–168), treated with Arthrobacter ureafaciens sialidase (Roche, Indianapolis, IN, http://www.roche.com; catalog no. 10269611001, 1 μl) in PBST at 37°C overnight and incubated with high-density lectin microarray at 20°C overnight (supplemental online Fig. 3). After washing with probing buffer (25 mM Tris-HCl, pH 7.5, 140 mM NaCl [TBS], 2.7 mM KCl, 1 mM CaCl2, 1 mM MnCl2, and 1% Triton X-100), the array was blocked with 60 μl of rabbit normal IgG (50 μg/ml) at 20°C for 1 hour. After washing again with probing buffer, the array was reacted with biotinylated goat anti-podocalyxin pAb (R&D; catalog no. AF1658) for 1 hour at 20°C. After a further wash with probing buffer, bound anti-podocalyxin pAb was detected with 1 μg/ml of Cy3-labeled streptavidin at 20°C for 30 minutes. After washing with probing buffer, fluorescence images were acquired using an evanescent field-activated fluorescence scanner (GlycoStation Reader 1200; GP BioSciences, Sapporo, Japan, http://www.gpbio.jp/english/index.html). The fluorescence signal of each spot was quantified using Array Pro Analyzer ver.4.5 (Media Cybernetics, Bethesda, MD, http://www.mediacy.com), and the background value was subtracted. The lectin signals of triplicate spots were averaged and normalized to the mean value of 96 lectins immobilized on the array to adjust the data from each microarray to account for possible systematic variation [20, 25].
Gene Expression Analysis
Total RNA was extracted from each cell sample using ISOGEN (Nippon Gene, Tokyo, Japan, http://www.nippongene.com). Global gene expression patterns were monitored using whole human genome microarray chips (G4112F; Agilent Technologies, Palo Alto, CA, http://www.agilent.com) with one-color (Cyanine 3) dye. Hybridization was determined with a G2505C microarray scanner system (Agilent). The data were analyzed using GeneSpring GX12.0 software (Agilent). Each chip was normalized to the 75th percentile of measurement taken from the chip.
Frontal Affinity Chromatography
The principle and protocol of frontal affinity chromatography (FAC) have been described previously [26, 27]. rBC2LCN was immobilized onto NHS-activated Sepharose 4FF (GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com), packed into a miniature column (inner diameter, 2 mm; length, 10 mm; bed volume, 31.4 μl; Shimadzu, Kyoto, Japan, http://www.shimadzu.com), and connected to a high-performance liquid chromatograph (Shimadzu). Pyridylaminated (PA) glycans prepared from human iPS cells (201B7) were injected into the column. The elution profile was then detected by fluorescence (excitation, 285 nm; emission, 350 nm). The elution front of PA glycan relative to that of negative control PA glycan (Manα1–6(Manα1–3)Manβ1–4GlcNAcβ1–4GlcNAc-PA), referred to as V-V0, was then determined. Woolf-Hofstee plots were constructed using the V-V0 values. The intercept and slope of the fitted line represent Bt (nmol) and −Kd (μM), respectively. Analysis of concentration dependence was performed using globo H (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc)-p-nitrophenol.
Results
Podocalyxin Is a Candidate Ligand of rBC2LCN as Revealed by DNA Microarray
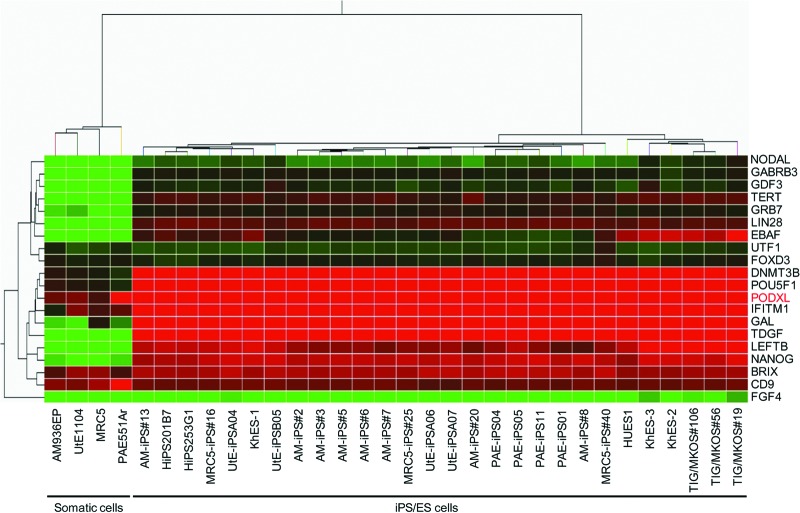
Using the high-density lectin microarray, we have previously shown that a probe lectin, rBC2LCN, exhibits rigorous specificity to undifferentiated human iPS cells and ES cells, but not at all to differentiated somatic cells [7]. Therefore, identification of glycoprotein ligands of rBC2LCN is critical to determine the molecular mechanism of undifferentiation from both developmental and glycobiological viewpoints. As a practical approach, we focused our attention to a candidate glycoprotein, podocalyxin, with respect to the following criteria: (a) highly expressed in human iPS cells and ES cells; (b) correlated with Nanog expression (pluripotency marker); (c) localized at the cell surface; (d) heavily glycosylated; and (e) antibodies used for immunoprecipitation are commercially available. In gene expression analysis of human iPS cells, ES cells, and somatic cells using DNA microarray, the podocalyxin gene was found to be highly expressed in all of the human iPS cells and ES cells (criteria a and b) (Fig. 1). High expression was also detected in one of the somatic cells derived from placental artery endothelial (PAE) and low but significant expression in other three somatic cells of MRC5, amniotic mesodermal (AM), and uterine endometrium (UtE) at the transcription level. Human podocalyxin is a type 1 membrane protein consisting of 528 amino acids, with criterion c thus satisfied given that the protein is localized at the cell surface [28]. The extracellular domain of podocalyxin has a mucin domain with a high serine/threonine content for putative O-glycosylation modifications. Podocalyxin also has five potential N-linked glycosylation sites and three putative glycosaminoglycan sites. Indeed, the apparent molecular weight has been reported to be ∼200 kDa in embryonic carcinoma cells [29], despite the calculated molecular weight of 55 kDa, demonstrating that human podocalyxin is heavily glycosylated (criterion d). Various types of polyclonal and monoclonal antibodies raised against the extracellular and the intracellular domains of human podocalyxin are commercially available (criterion e).
Figure 1.
Clustering of human stem cell markers versus cell lines. The heat map shows a two-way cluster analysis carried out on the data of the cell lines listed in supplemental online Table 1. The data were obtained as averages of multiple cell samples. Levels of gene expression are indicated by color changes from red (high expression levels) to green (low expression levels). Podocalyxin expression is indicated as “PODXL” in red. Abbreviations: ES, embryonic stem; iPS, induced pluripotent stem.
High Molecular Weight Proteins of >240 kDa Are the Glycoprotein Ligands of rBC2LCN in Human iPS Cells and ES Cells
To search glycoprotein ligands of rBC2LCN in human iPS cells and ES cells, hydrophilic fractions of human iPS cells and ES cells in addition to mouse feeder cells and somatic cells were first separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted with HRP-conjugated rBC2LCN. Representative iPS cells prepared from various tissue types (MRC5-iPS25 [P22] [18], AM-iPS3 [P7] [29], UtE-iPSA04 [P27] [71], PAEiPS01 [P26] [95], TIG/MKOS19 [P37] [141], HiPS201B7 [P45] [123], hiPS253G1 [P32] [128]) and ES cell lines (KhES-1 [P26] [156], KhES-2 [P31] [159], KhES-3 [P28] [160], HUES1 [163]) were randomly selected among 138 types of iPS cells and 15 types of ES cells (supplemental online Table 1) and used for this analysis. As shown in Figure 2, rBC2LCN bound to all of the undifferentiated iPS cells and ES cells tested, but not at all to differentiated mouse feeder cells (MEFs) (no. 1) and somatic cells of the iPS origin (MRC5 [no. 2], AM936EP [no. 5], UtE1104 [no. 9], PAE551Ar [P16] [no. 11], and h-Fib [no. 13]), supporting the previous report that rBC2LCN is highly specific to undifferentiated cells [7]. In iPS cells and ES cells, strong and diffuse protein bands were detected at a high molecular weight of >240 kDa, whereas weaker bands were also detected from 70 to 140 kDa, indicating that other glycoprotein ligands of rBC2LCN are also present in human iPS and ES cells.
Figure 2.
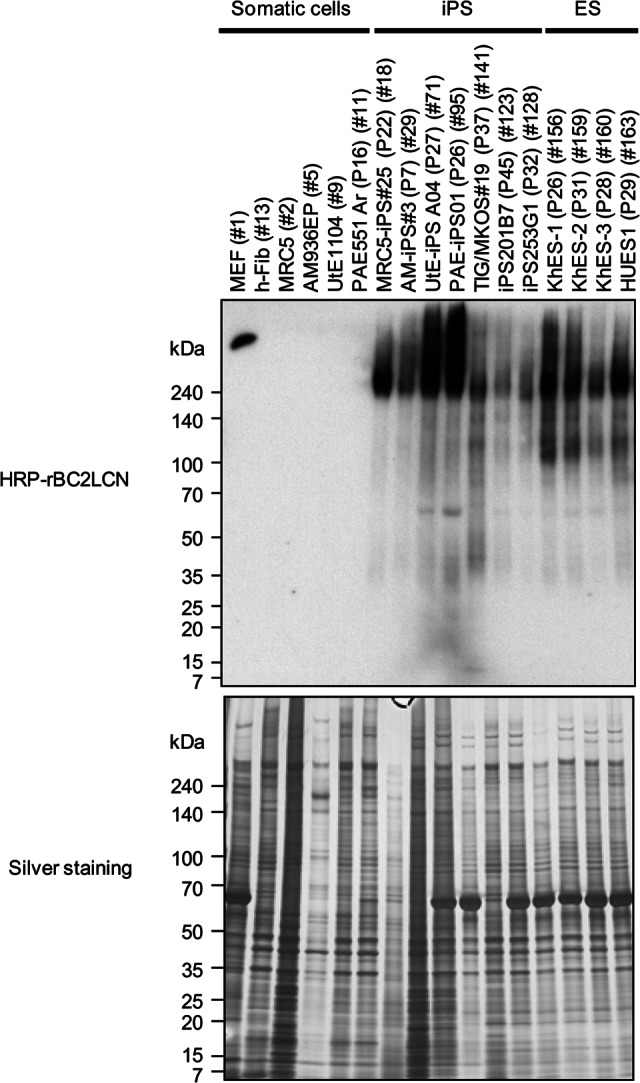
High molecular weight proteins of >240 kDa are glycoprotein ligands of rBC2LCN in human iPS cells and ES cells. One microgram of hydrophilic fractions of somatic cells, iPS cells, and ES cells was run on 5%–20% acrylamide gel under reducing conditions, electroblotted onto polyvinylidene difluoride membrane, and stained with 1 μg/ml of HRP-conjugated rBC2LCN (top panel). Silver staining was also performed (bottom panel). Abbreviations: ES, embryonic stem; HRP, horseradish peroxidase; iPS, induced pluripotent stem; MEF, mouse embryonic fibroblasts.
Podocalyxin Is a Glycoprotein Ligand of rBC2LCN
The molecular nature of the rBC2LCN-positive bands such as a high molecular weight and high expression in human iPS cells and ES cells strongly suggested that podocalyxin is a glycoprotein ligand of rBC2LCN. Streptavidin-coated magnetic beads immobilized with biotinylated goat anti-podocalyxin pAb were incubated overnight at 4°C with hydrophilic fractions of iPS cells. After extensive washing of the beads, bound fractions were eluted with TBS containing 0.2% SDS at 95°C for 10 minutes. The elution fractions were then separated by SDS-PAGE and blotted with goat anti-podocalyxin pAb followed by HRP-labeled donkey anti-goat IgG. As shown in Figure 3 (bottom panel), podocalyxin could be immunologically detected as a diffuse high molecular weight band at >240 kDa in the immunoprecipitates from human iPS cells and ES cells, but not those from mouse feeder cells and somatic cells. Because the calculated molecular weight of podocalyxin is 55 kDa, it is evident that podocalyxin, expressed in human iPS cells and ES cells, is heavily glycosylated. Indeed, rBC2LCN exhibited strong binding to the immunoprecipitated podocalyxin as a diffuse protein band at >240 kDa in human iPS cells and ES cells (Fig. 3, top panel). Stronger staining was observed with rBC2LCN relative to anti-podocalyxin pAb. The rBC2LCN reactivity varied among the cell types of iPS cells and ES cells. Stronger signals were observed for UtE-iPS A04 (P27) (71) and PAE-iPS01 (P26) (95), whereas weaker signals were detected for TIG/MKOS19 (P37) (141), iPS201B7 (P45) (123), and iPS253G1 (P32) (128). Although the podocalyxin gene was detected in PAE551Ar, the corresponding podocalyxin protein was not detected in the anti-podocalyxin immunoprecipitates (Fig. 3), indicating that the podocalyxin expression is restricted to undifferentiated cells at the translation level among the cell types used in this study (supplemental online Table 1). It should be noted that podocalyxin could be precipitated from hydrophilic but not hydrophobic fractions (data not shown), even though podocalyxin is basically a transmembrane protein. This can be attributed, at least in part, to the highly hydrophilic nature of podocalyxin, which is heavily glycosylated. Altogether, these results clearly demonstrate that podocalyxin is a glycoprotein ligand of rBC2LCN, although the possibility that other glycoprotein ligands with >240 kDa are included remains.
Figure 3.
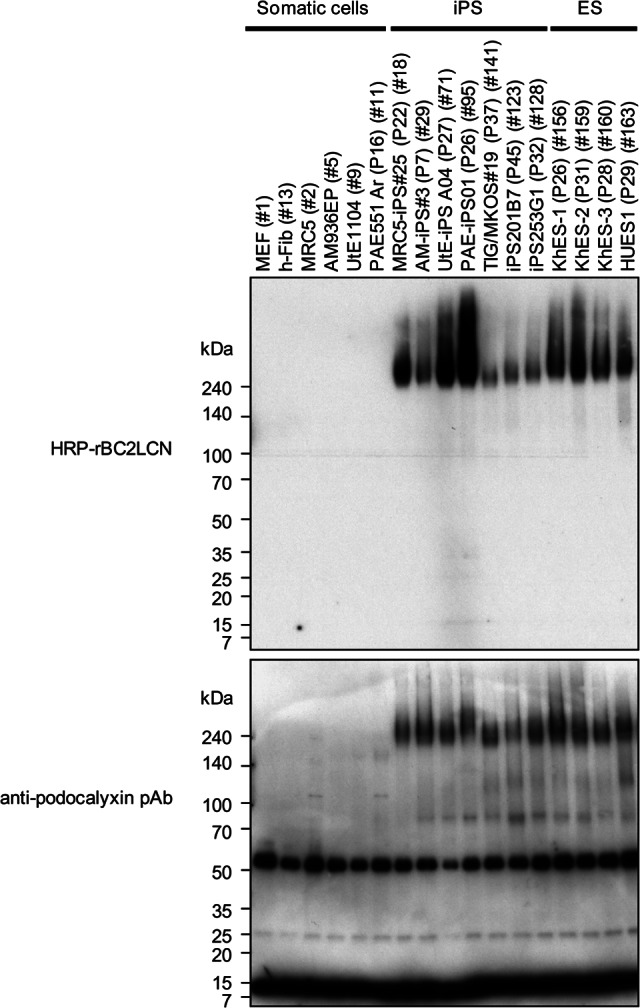
Podocalyxin is a glycoprotein ligand of rBC2LCN. Dynabeads M-280 streptavidin (10 μl) immobilized with biotinylated anti-podocalyxin pAb (1 μg) was incubated with hydrophilic fractions of cells at 4°C overnight with agitation. After washing the beads with 200 μl of PBST three times, bound podocalyxin was eluted with 50 μl of TBS containing 0.2% SDS at 95°C for 10 minutes. Coeluted biotinylated anti-podocalyxin pAb was partially depleted with Dynabeads M-280 streptavidin (15 μl) at room temperature for 1 hour with agitation. The eluted fractions were run on 5%–20% acrylamide gel under reducing condition and transferred to polyvinylidene difluoride membrane. The membrane was stained with either HRP-conjugated rBC2LCN (1 μg/ml, top panel) or goat anti-podocalyxin pAb (0.1 μg/ml) followed by HRP-conjugated donkey anti-goat IgG (×10,000, bottom panel). The staining was performed in different membranes. Both 55- and 10-kDa bands are nonspecific, since both bands could be detected in the control immunoprecipitated samples. The 55-kDa band might be the heavy chain of goat anti-podocalyxin pAb used for the immunoprecipitation of podocalyxin, whereas the identity of the 10-kDa band is unknown. Abbreviations: ES, embryonic stem; HRP, horseradish peroxidase; iPS, induced pluripotent stem; MEF, mouse embryonic fibroblasts; pAb, polyclonal antibody.
Podocalyxin Is a Glycoprotein Ligand of rBC2LCN Common to Human iPS Cells and ES Cells Revealed by Antibody-Overlay Lectin Microarray
To examine whether rBC2LCN binds to human podocalyxin derived from human pluripotent stem cells of more than 100 samples in a high-throughput manner, we applied the recently developed high-throughput technology called antibody-overlay lectin microarray, which allows interaction analysis between immobilized lectins and a nanogram order of target samples [10]. A schematic representation of the technique is shown in supplemental online Figure 3. The human podocalyxin immunoprecipitated from human iPS cells and ES cells was reacted with rBC2LCN immobilized on a glass slide [7, 23]. After blocking of nonspecific binding sites with normal rabbit IgG, bound podocalyxin was selectively visualized with biotinylated anti-podocalyxin pAb followed by Cy3-labeled streptavidin, according to the established protocol [10]. The binding signals were detected and quantified using an evanescent-field fluorescence-assisted scanner as described [23]. To further confirm this, the immunoprecipitated podocalyxin was pretreated with A. ureafaciens sialidase before application to the lectin microarray, because this treatment was found to enhance the interaction between podocalyxin and the immobilized rBC2LCN. This could be explained in part by reduced electric repulsion caused by the strong negative charge of the heavily sialylated podocalyxin [30]. Using the advanced high-throughput technology, a series of cell samples including 138 types of human iPS cells prepared from six different origins with various passages (16–153 in supplemental online Table 1) and 15 types of human ES cells (154–168) were analyzed [20]. For reference (negative control), mouse feeder cells (MEFs) (1) and differentiated somatic cells of the iPS origin (2–15) were also examined. Figure 4A shows the results of rBC2LCN binding for representative samples, that is, MEFs (1), fibroblasts (166), iPS cells (115), and ES cells (154), whereas Figure 4B provides a bar graph representation of the total analysis. It was verified unambiguously that all of the iPS cells and ES cells examined bound to rBC2LCN, although the binding degrees were significantly varied. On the other hand, no detectable signal was observed for rBC2LCN binding to feeder (1) or somatic cells (2–15) as expected, since podocalyxin was not immunoprecipitated from these cell samples (Fig. 3). These results confirm the fact that podocalyxin is a universal glycoprotein ligand of rBC2LCN in human undifferentiated pluripotent cells.
Figure 4.
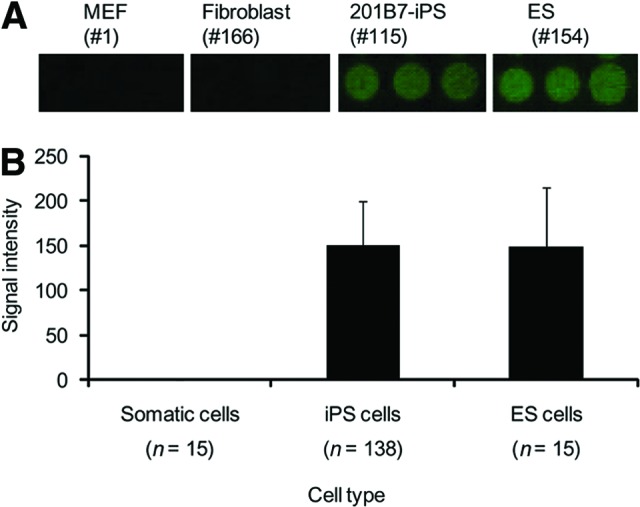
Podocalyxin is a glycoprotein ligand common to human iPS cells and ES cells. Podocalyxin was immunoprecipitated from hydrophobic fractions with streptavidin-coated magnetic beads immobilizing biotinylated anti-podocalyxin polyclonal antibody (pAb). The elution fraction was pretreated with A. ureafaciens sialidase and incubated with high-density lectin microarray containing rBC2LCN. After blocking with rabbit IgG, the array was incubated with biotinylated anti-podocalyxin pAb followed by Cy3-labeled streptavidin and scanned with an evanescent-field fluorescence scanner. Representative binding images obtained with a 120× gain on triplicate spots of rBC2LCN are shown in (A). The fluorescence signal of each spot was quantified using Array Pro Analyzer version 4.5, and the background value was subtracted. The lectin signals of triplicate spots were averaged and normalized to the mean value of 96 lectins immobilized on the array. The data are shown as the averages ± S.D. of the binding of rBC2LCN to podocalyxin immunoprecipitated from somatic cells (n = 14; 2–15 in supplemental online Table 1), iPS cells (n = 138; 16–153), and ES cells (n = 15; 154–168) (B). Abbreviations: ES, embryonic stem; iPS, induced pluripotent stem; MEF, mouse embryonic fibroblasts.
Glycan Ligands on Podocalyxin Recognized by rBC2LCN Are O-Glycans
Having demonstrated that podocalyxin is a substantial glycoprotein ligand of rBC2LCN both in iPS cells and ES cells, we next analyzed the glycan structures on podocalyxin and the classes of glycans to which rBC2LCN binds. For this purpose, we first performed antibody-overlay lectin microarray assisted with enzyme treatments (supplemental online Fig. 4). Since podocalyxin has been reported to contain N- and O-glycans, and keratan sulfate glycosaminoglycans [4, 28, 31–33], the protein was digested with PNGase F, an amidase that cleaves N-glycans between the innermost GlcNAc and asparagine residues of N-linked glycoproteins, and with keratanase II from Bacillus sp. Ks36, an endo-β-N-acetylglycosaminidase that hydrolyzes keratan sulfate between the 4GlcNAcβ1–3Galβ1 structure [34]. Before the enzyme treatments, podocalyxin was heat-denatured and treated with A. ureafaciens sialidase to increase the enzyme susceptibility. Podocalyxin immunoprecipitated from TIG/MKOS19 (P50) chosen as a representative iPS cell line was treated with either sialidase, sialidase and PNGase F, or sialidase and keratanase II and incubated with high-density lectin microarray. Bound podocalyxin was analyzed by the antibody-overlay method as described above. As shown in supplemental online Figure 4, the signals for SNA (Sambucus nigra agglutinin), an α2–6Sia-binding lectin, were significantly decreased after the sialidase treatment, demonstrating that podocalyxin is α2–6sialylated. The residual signals are attributable to either the incomplete sialidase digestion or the binding to asialo N-glycans, because SNA also shows weak but significant binding to LacNAc (Galβ1–4GlcNAc) [35]. With the sialidase and PNGase F double treatments, the binding of a high-mannose-type N-linked glycan-binding lectin (recombinant griffithsin [rGRFT]) was also decreased. This indicates that podocalyxin carries high-mannose type N-linked glycans [32]. The binding of Psathyrella velutina lectin (PVL) with specificity to nonsubstituted βGlcNAc and 6-O-substituted βGlcNAc, including 6S-GlcNAc at the nonreducing end [36, 37] was decreased upon double treatments with sialidase and keratanase II. This observation supports a previous report that podocalyxin contains keratan sulfate, which is susceptible to keratanase II treatment [4]. The results also suggest that keratan sulfate is largely attributed to O-glycans, since the PVL signal was not susceptible to PNGase F treatment. In contrast, the signals of an O-glycan binder (Amaranthus caudatus lectin [ACA]) were increased after sialidase treatment and further enhanced with sialidase and keratanase II double treatments. Increased signals can be explained by the increased accessibility of this O-glycan binding lectin caused by the decreased charge repulsion and/or steric hindrance caused by heavy sialylation and sulfation of podocalyxin. Furthermore, significant signals of the α1–2fucose-binding lectin MCA could be observed, which were also increased upon enzyme treatments. Similarly, the signals of rBC2LCN were increased after sialidase treatment and further enhanced with the sialidase and keratanase II double treatment, leading to the hypothesis that the major carbohydrate antigens of rBC2LCN are O-glycans.
To prove this hypothesis, we further performed a lectin blotting experiment. The podocalyxin immunoprecipitated from iPS cells (iPS201B7 [P45], 123) and ES cells (KhES-1 [P26], 156) was treated with or without PNGase F, run on SDS-PAGE, and blotted onto PVDF membrane. The membrane was then treated with or without 0.05 M NaOH at 40°C for 16 h to remove O-glycans by β-elimination [21, 22] and analyzed by lectin blotting using HRP-conjugated rBC2LCN. As shown in Figure 5, most rBC2LCN signals were still left upon PNGase F treatment, whereas the alkaline hydrolysis greatly reduced the rBC2LCN binding, demonstrating that the carbohydrate antigens of rBC2LCN are expressed on O-glycans of podocalyxin prepared from human iPS cells and ES cells. Since the alkaline treatment gave essentially no effect on the rBC2LCN binding to a positive control neoglycoprotein (Fucα1–2Galβ1–3GlcNAcβ1–3Galβ1–4Glc-BSA), the reduced staining is not due to the loss of the blotted proteins.
Figure 5.
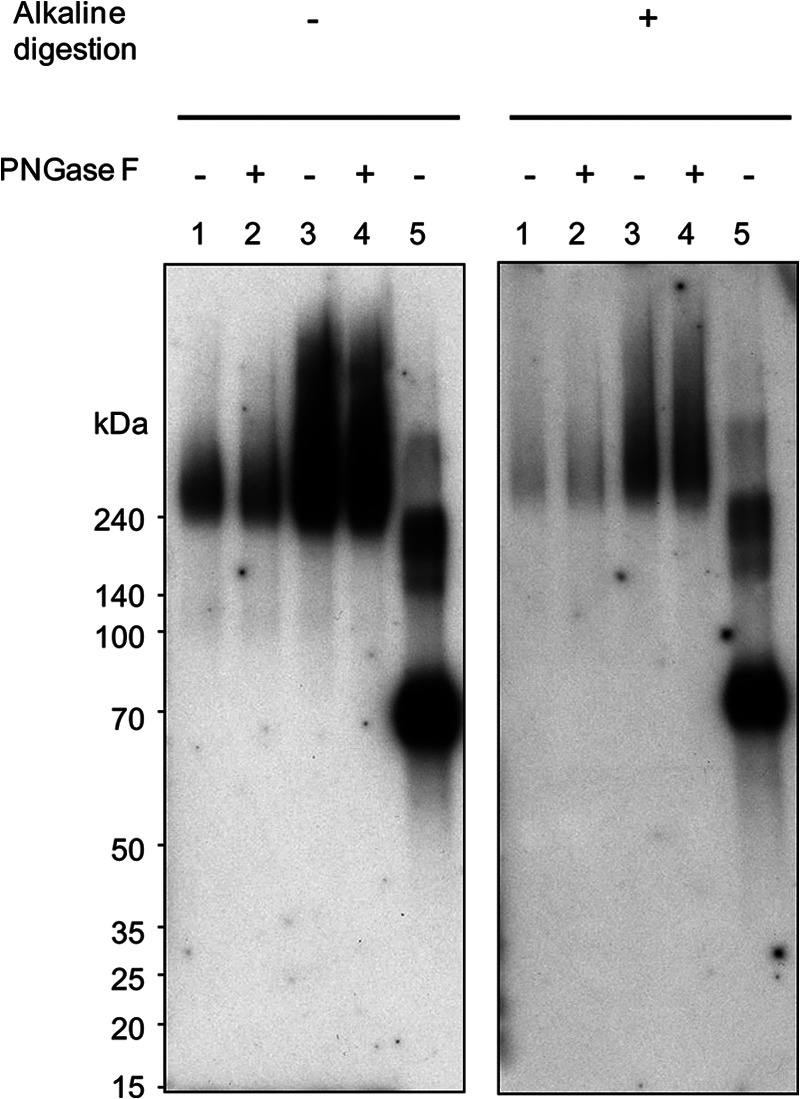
Glycan ligands on podocalyxin recognized by rBC2LCN are O-glycans. The podocalyxin immunoprecipitated from induced pluripotent stem cells (iPS201B7 [P45], 123; lanes 1 and 2) and embryonic stem cells (KhES-1 [P26], 156; lanes 3 and 4) with or without PNGase F treatment was run on 5%–20% acrylamide gel under reducing condition and transferred onto polyvinylidene difluoride membranes. The membranes were treated with phosphate-buffered saline (left panel) or 0.05 M NaOH (right panel) and blotted with horseradish peroxidase-conjugated rBC2LCN. Lane 5 shows Fucα1–2Galβ1–3GlcNAcβ1–3Galβ1–4Glc-BSA (rBC2LCN ligand, 0.5 μg). Abbreviation: PNGase F, Peptide:N-glycosidase F.
rBC2LCN Recognizes an O-Glycan Containing an H Type 3 Isolated From iPS Cells
Recently, we performed quantitative glycome analysis targeting both N- and O-glycans of undifferentiated iPS cells (201B7) and differentiated human dermal fibroblasts by a glycosidase-assisted high-performance liquid chromatography (HPLC) method combined with mass spectrometry [38]. Among the 37 types of N-glycans and 10 types of O-glycans identified from iPS cells, one O-glycan with an m/z value of 973.4 [M+H]+ containing an H type 3 (Fucα1–2Galβ1–3GalNAc) was isolated, of which structure was identified to be Fucα1–2Galβ1–3(Galβ1–3GlcNAcβ1–6)GalNAc-PA by HPLC mapping assisted with matrix-assisted laser desorption ionization time-of-flight mass spectrometry and exoglycosidase digestion analyses [38], where “PA” represents a reducing terminal pyridylamino group. On the other hand, no glycan containing H type 1 was detected. Thus, we determined the association constant between rBC2LCN and this O-glycan carrying an H type 3 structure (hereafter designated glycan a) using a quantitative frontal affinity chromatography (FAC) technique [26] (Fig. 6). For comparison, its isomeric O-glycan containing an H type 2 structure (Fucα1–2Galβ1–4GlcNAcβ1–6(Galβ1–3)GalNAc-PA, designated glycan b), was analyzed, which was also prepared from 201B7 iPS cells. As a result, rBC2LCN was found to bind glycan a containing the H type 3 structure with a Ka of 2.5 × 104 M−1, whereas no detectable binding was observed for the related glycan b containing the H type 2 structure (Ka < 6.7 × 103 M−1). Consistent with a recent study using glycan microarray [7], the present FAC analysis using 126 standard PA glycans confirmed that rBC2LCN bound to H type 1 (Fucα1–2Galβ1–3GlcNAcβ1–3Galβ1–4Glc-PA, Ka = 2.8 × 104 M−1) and Leb (Fucα1–2Galβ1–3(Fucα1–4)GlcNAcβ1–3Galβ1–4Glc-PA, Ka = 2.0 × 105 M−1), but not to other glycans without the defined epitope structure Fucα1–2Galβ1–3GlcNAc(GalNAc). They include high-mannose-type, agalactosylated, galactosylated, sialylated N-glycans, and glycolipid-type glycans, demonstrating that rBC2LCN is highly specific to this glycan epitope (data not shown). Combined with the facts that podocalyxin is heavily O-glycosylated on its mucin domain and that podocalyxin binding to rBC2LCN was decreased by an alkaline treatment, it is most likely that the carbohydrate antigens on podocalyxin recognized by rBC2LCN are H type 3-containing O-glycans, such as glycan a isolated from iPS201B7 cells.
Figure 6.
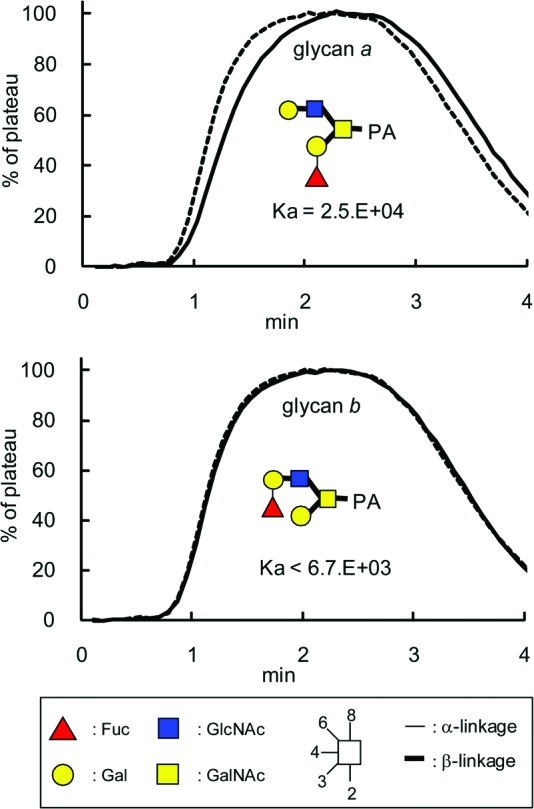
rBC2LCN binds to an O-glycan containing H type 3 isolated from induced pluripotent stem (iPS) cells. rBC2LCN was immobilized onto NHS-activated Sepharose 4FF (GE Healthcare) and packed into a miniature column and connected to a high-performance liquid chromatograph. PA glycans (glycans a and b, solid lines) prepared from human iPS cells (201B7) were injected into the column. The elution profile was then detected by fluorescence (excitation, 285 nm; emission, 350 nm). The elution front of PA glycan relative to that of negative control PA glycan (Manα1–6(Manα1–3)Manβ1–4GlcNAcβ1–4GlcNAc-PA, dotted line), referred to as V-V0, was then determined. Woolf-Hofstee plots were prepared using the V-V0 value. The intercept and slope of the fitted line represent Bt (nmol) and −Kd (μM), respectively. Analysis of concentration dependence was performed using globo H (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc)-p-nitrophenol. Abbreviation: PA, pyridylaminated.
Discussion
Podocalyxin is a type I transmembrane sialoglycoprotein of the CD34 family, which was originally cloned from the human kidney as a component of the podocyte cell glycocalyx [28]. Later, podocalyxin was reported to act as a pluripotent stem cell marker, which is highly expressed on human ES cells [39–42]. Subsequently, podocalyxin was identified as a carrier molecule of the well-known pluripotency stem cell markers TRA-1-60 and TRA-1-81 [4]. In this study, we have clearly demonstrated that podocalyxin is a glycoprotein ligand of rBC2LCN, a novel probe for human pluripotent cells [7]. This indicates a possibility that podocalyxin functions as a ligand of both probes (TRA-1-60/81 and rBC2LCN) specific to human pluripotent stem cells.
In lectin-blotting analysis, rBC2LCN bound exclusively to undifferentiated human iPS cells and ES cells, but not at all to differentiated somatic cells, supporting the previous finding that rBC2LCN is highly specific to pluripotent stem cells [7]. Surprisingly, podocalyxin was found to be the major glycoprotein ligand of rBC2LCN in human iPS cells and ES cells. However, this could be explained by the unique molecular nature of podocalyxin, that is, highly expressed in pluripotent stem cells and heavily glycosylated, whereas altered glycosylation machinery upon induction of pluripotency should affect various glycoproteins rather than a specific one. In fact, rBC2LCN-positive bands were detected from 70 to 140 kDa (Fig. 2), but their contribution is rather small compared with podocalyxin. Notably, the podocalyxin protein was detectable at a similar degree in iPS cells and ES cells, whereas the rBC2LCN reactivity was varied among the cell types of iPS cells and ES cells. In this context, it is interesting to speculate that the rBC2LCN reactivity might correlate with the degree of differentiation of pluripotent stem cells.
Alkaline digestion, but not PNGase F treatment, reduced the binding of rBC2LCN to podocalyxin, indicating that the carbohydrate antigens on podocalyxin are presented partly on O-glycans. Previously, we have performed quantitative glycome analysis targeting both N- and O-glycans of undifferentiated iPS cells (201B7) and differentiated human dermal fibroblasts by a glycosidase-assisted HPLC method combined with mass spectrometry [38]. Among 47 types of N- and O-glycans identified from iPS cells, a potential carbohydrate antigen of rBC2LCN was identified, with an O-glycan-carrying H type 3 epitope structure (Fucα1–2Galβ1–3(Galβ1–3GlcNAcβ1–6)GalNAc-PA), whereas H type 1 could not be detected on either N- and O-glycans. Indeed, rBC2LCN bound to this O-glycan with a Ka of 2.5 × 104 M−1. Even though the binding affinity of rBC2LCN to glycan a is relatively weak (Ka of 2.5 × 104 M−1), the affinity to podocalyxin is expected to be increased, because of the high density of the glycan ligands expressed on podocalyxin by a so-called “glycan cluster effect” [43]. Altogether, these results indicate that H type 3 is a most probable human pluripotency marker, which is expressed exclusively in undifferentiated iPS cells [38] and is recognized by rBC2LCN.
Podocalyxin has important roles in cell morphology, adhesion, and migration in a variety of tissues including kidney podocytes, hematopoietic progenitors, vascular endothelia, and a subset of neurons [44]. In the developing kidney, podocalyxin plays an essential role in the formation and maintenance of podocyte foot processes [44]. Podocalyxin-null mice fail to form foot processes and slit diaphragms and thus exhibit profound defects in kidney development and die within 24 hours of birth with anuric renal failure [45]. The ectopic expression of podocalyxin induces morphologic changes including actin recruitment and the formation of microvillus and foot process in a manner dependent of its extracellular domain [44]. Podocalyxin coats the secondary foot processes of the podocytes and functions to open the urinary filtration barrier by keeping adjacent foot processes separated by its negative charge. Podocalyxin is also expressed in the hematopoietic system and is involved in cell migration [44]. In addition, podocalyxin is abnormally expressed in subsets of breast, prostate, liver, pancreatic, and kidney cancer, as well as leukemia, and is likely involved in metastasis [44]. Although the biological functions of podocalyxin in stem cells and its relationship with pluripotency are largely unknown, it is likely that podocalyxin regulates the maintenance and morphology of stem cells as well [4]. It would be of interest to investigate how changes of glycan structures on podocalyxin affect its protein and/or cellular functions. Indeed, partial loss-of-function mutation in the gene encoding glycoprotein-N-acetylgalactosamine-3-β-galactosyltransferase (C1GalT1), an enzyme essential for the synthesis of core1 (Galβ1–3GalNAc), which is the precursor of H type 3 (Fucα1–2Galβ1–3GalNAc), was reported to cause kidney diseases including distortion of the glomerular-tubular architecture [46]. In these mice, podocalyxin is the major underglycosylated protein, and its appropriate glycosylation was implicated to be essential for kidney functions. Therefore, it is likely that altered glycosylation affects both the chemical and physical properties of podocalyxin as well as its recognition molecules, leading to the modification and regulation of cell-cell communications, which is important for the maintenance of pluripotency.
Conclusion
We conclude that podocalyxin is the glycoprotein ligand of rBC2LCN in human iPS and ES cells. The carbohydrate antigens of rBC2LCN are expressed on O-glycans of podocalyxin, since alkaline hydrolysis greatly reduced the binding of rBC2LCN to human iPS cells and ES cells. rBC2LCN bound to an O-glycan carrying H type 3 epitope structure isolated from iPS cells, suggesting that H type 3 is a novel pluripotency glycan marker. Further studies will be crucial to understand the roles of glycans of podocalyxin for pluripotency and self-renewal of human iPS cells and ES cells.
Acknowledgments
We thank Dr. Atsushi Kuno and Dr. Yoko Itakura for advice and discussion and Yoshiko Kubo, Jinko Murakami, Yasuhiko Aiki, and Dr. Kumiko Higuchi for technical assistance. Dr. Hiromi Ito provided the globo H (Fucα1–2Galβ1–3GalNAcβ1–3Galα1–4Galβ1–4Glc)-p-nitrophenyl. This work was supported in part by the New Energy and Industrial Technology Development Organization in Japan. Human iPS cells (201B7) were obtained from RIKEN Bioresource Center. K.N. is currently affiliated with the Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Author Contributions
H.T.: conception and design, manuscript writing, data analysis and interpretation; A.M., K. Hiemori, and K. Hasehira: data analysis and interpretation; Y.O., Y. Ito, K.N., M.O., S.T., Mahito Nakanishi, Y. Ikehara, Mio Nakanishi, K.O., T.C., M.T., H.A., and A.U.: provision of study material or patients; M.A.: provision of study material or patients, financial support; J.H.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Muramatsu T, Muramatsu H. Carbohydrate antigens expressed on stem cells and early embryonic cells. Glycoconj J. 2004;21:41–45. doi: 10.1023/B:GLYC.0000043746.77504.28. [DOI] [PubMed] [Google Scholar]
- 2.Andrews PW. Toward safer regenerative medicine. Nat Biotechnol. 2011;29:803–805. doi: 10.1038/nbt.1974. [DOI] [PubMed] [Google Scholar]
- 3.Lanctot PM, Gage FH, Varki AP. The glycans of stem cells. Curr Opin Chem Biol. 2007;11:373–380. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schopperle WM, DeWolf WC. The TRA-1-60 and TRA-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells. 2007;25:723–730. doi: 10.1634/stemcells.2005-0597. [DOI] [PubMed] [Google Scholar]
- 5.Natunen S, Satomaa T, Pitkanen V, et al. The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency associated type 1 lactosamine epitope. Glycobiology. 2011;21:1125–1130. doi: 10.1093/glycob/cwq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang C, Lee AS, Volkmer JP, et al. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tateno H, Toyota M, Saito S, et al. Glycome diagnosis of human induced pluripotent stem cells using lectin microarray. J Biol Chem. 2011;286:20345–20353. doi: 10.1074/jbc.M111.231274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulák O, Cioci G, Delia M, et al. A TNF-like trimeric lectin domain from Burkholderia cenocepacia with specificity for fucosylated human histo-blood group antigens. Structure. 2010;18:59–72. doi: 10.1016/j.str.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Onuma Y, Tateno H, Hirabayashi J, et al. rBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem Biophys Res Commun. 2013;431:524–529. doi: 10.1016/j.bbrc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Kuno A, Kato Y, Matsuda A, et al. Focused differential glycan analysis with the platform antibody-assisted lectin profiling for glycan-related biomarker verification. Mol Cell Proteomics. 2009;8:99–108. doi: 10.1074/mcp.M800308-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Nishino K, Toyoda M, Yamazaki-Inoue M, et al. Defining hypo-methylated regions of stem cell-specific promoters in human iPS cells derived from extra-embryonic amnions and lung fibroblasts. PLoS One. 2010;5:e13017. doi: 10.1371/journal.pone.0013017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata S, Toyoda M, Yamaguchi S, et al. Efficient reprogramming of human and mouse primary extra-embryonic cells to pluripotent stem cells. Genes Cells. 2009;14:1395–1404. doi: 10.1111/j.1365-2443.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Makino H, Toyoda M, Matsumoto K, et al. Mesenchymal to embryonic incomplete transition of human cells by chimeric OCT4/3 (POU5F1) with physiological co-activator EWS. Exp Cell Res. 2009;315:2727–2740. doi: 10.1016/j.yexcr.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Toyoda M, Yamazaki-Inoue M, Itakura Y, et al. Lectin microarray analysis of pluripotent and multipotent stem cells. Genes Cells. 2011;16:1–11. doi: 10.1111/j.1365-2443.2010.01459.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito S, Onuma Y, Ito Y, et al. Possible linkages between the inner and outer cellular states of human induced pluripotent stem cells. BMC Syst Biol. 2011;5(suppl 1):S17. doi: 10.1186/1752-0509-5-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura K, Sano M, Ohtaka M, et al. Development of defective and persistent Sendai virus vector: A unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286:4760–4771. doi: 10.1074/jbc.M110.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suemori H, Yasuchika K, Hasegawa K, et al. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem Biophys Res Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- 19.Osafune K, Caron L, Borowiak M, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 20.Tateno H, Kuno A, Itakura Y, et al. A versatile technology for cellular glycomics using lectin microarray. Methods Enzymol. 2010;478:181–195. doi: 10.1016/S0076-6879(10)78008-3. [DOI] [PubMed] [Google Scholar]
- 21.Duk M, Ugorski M, Lisowska E. Beta-Elimination of O-glycans from glycoproteins transferred to immobilon P membranes: Method and some applications. Anal Biochem. 1997;253:98–102. doi: 10.1006/abio.1997.9994. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y, Hayatsu N, Kaneko MK, et al. Increased expression of highly sulfated keratan sulfate synthesized in malignant astrocytic tumors. Biochem Biophys Res Commun. 2008;369:1041–1046. doi: 10.1016/j.bbrc.2008.02.130. [DOI] [PubMed] [Google Scholar]
- 23.Kuno A, Uchiyama N, Koseki-Kuno S, et al. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat Methods. 2005;2:851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama N, Kuno A, Tateno H, et al. Optimization of evanescent-field fluorescence-assisted lectin microarray for high-sensitivity detection of monovalent oligosaccharides and glycoproteins. Proteomics. 2008;8:3042–3050. doi: 10.1002/pmic.200701114. [DOI] [PubMed] [Google Scholar]
- 25.Itakura Y, Nakamura-Tsuruta S, Kominami J, et al. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: A search by frontal affinity chromatography. J Biochem. 2007;142:459–469. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- 26.Tateno H, Nakamura-Tsuruta S, Hirabayashi J. Frontal affinity chromatography: Sugar-protein interactions. Nat Protoc. 2007;2:2529–2537. doi: 10.1038/nprot.2007.357. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura S, Yagi F, Totani K, et al. Comparative analysis of carbohydrate-binding properties of two tandem repeat-type Jacalin-related lectins, Castanea crenata agglutinin and Cycas revoluta leaf lectin. FEBS J. 2005;272:2784–2799. doi: 10.1111/j.1742-4658.2005.04698.x. [DOI] [PubMed] [Google Scholar]
- 28.Kershaw DB, Beck SG, Wharram BL, et al. Molecular cloning and characterization of human podocalyxin-like protein: Orthologous relationship to rabbit PCLP1 and rat podocalyxin. J Biol Chem. 1997;272:15708–15714. doi: 10.1074/jbc.272.25.15708. [DOI] [PubMed] [Google Scholar]
- 29.Schopperle WM, Armant DR, DeWolf WC. Purification of a tumor-specific PNA-binding glycoprotein, gp200, from a human embryonal carcinoma cell line. Arch Biochem Biophys. 1992;298:538–543. doi: 10.1016/0003-9861(92)90447-5. [DOI] [PubMed] [Google Scholar]
- 30.Takeda T, Go WY, Orlando RA, et al. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerjaschki D, Vernillo AT, Farquhar MG. Reduced sialylation of podocalyxin—the major sialoprotein of the rat kidney glomerulus—in aminonucleoside nephrosis. Am J Pathol. 1985;118:343–349. [PMC free article] [PubMed] [Google Scholar]
- 32.Dekan G, Gabel C, Farquhar MG. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci USA. 1991;88:5398–5402. doi: 10.1073/pnas.88.12.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamagishi K, Suzuki K, Imai K, et al. Purification, characterization, and molecular cloning of a novel keratan sulfate hydrolase, endo-beta-N-acetylglucosaminidase, from Bacillus circulans. J Biol Chem. 2003;278:25766–25772. doi: 10.1074/jbc.M212183200. [DOI] [PubMed] [Google Scholar]
- 35.Yabe R, Itakura Y, Nakamura-Tsuruta S, et al. Engineering a versatile tandem repeat-type alpha2–6sialic acid-binding lectin. Biochem Biophys Res Commun. 2009;384:204–209. doi: 10.1016/j.bbrc.2009.04.090. [DOI] [PubMed] [Google Scholar]
- 36.Seko A, Ohkura T, Ideo H, et al. Novel O-linked glycans containing 6′-sulfo-Gal/GalNAc of MUC1 secreted from human breast cancer YMB-S cells: Possible carbohydrate epitopes of KL-6(MUC1) monoclonal antibody. Glycobiology. 2012;22:181–195. doi: 10.1093/glycob/cwr118. [DOI] [PubMed] [Google Scholar]
- 37.Kochibe N, Matta KL. Purification and properties of an N-acetylglucosamine-specific lectin from Psathyrella velutina mushroom. J Biol Chem. 1989;264:173–177. [PubMed] [Google Scholar]
- 38.Hasehira K, Tateno H, Onuma Y, et al. Structural and quantitative evidence for dynamic glycome shift upon production of human induced pluripotent stem cells. Mol Cell Proteomics. 2012;11:1913–1923. doi: 10.1074/mcp.M112.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards M, Tan SP, Tan JH, et al. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharya B, Miura T, Brandenberger R, et al. Gene expression in human embryonic stem cell lines: Unique molecular signature. Blood. 2004;103:2956–2964. doi: 10.1182/blood-2003-09-3314. [DOI] [PubMed] [Google Scholar]
- 41.Zeng X, Miura T, Luo Y, et al. Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cells. 2004;22:292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]
- 42.Hayman MW, Przyborski SA. Proteomic identification of biomarkers expressed by human pluripotent stem cells. Biochem Biophys Res Commun. 2004;316:918–923. doi: 10.1016/j.bbrc.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 43.Dam TK, Gerken TA, Brewer CF. Thermodynamics of multivalent carbohydrate-lectin cross-linking interactions: Importance of entropy in the bind and jump mechanism. Biochemistry. 2009;48:3822–3827. doi: 10.1021/bi9002919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen JS, McNagny KM. The role of podocalyxin in health and disease. J Am Soc Nephrol. 2009;20:1669–1676. doi: 10.1681/ASN.2008070782. [DOI] [PubMed] [Google Scholar]
- 45.Doyonnas R, Kershaw DB, Duhme C, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001;194:13–27. doi: 10.1084/jem.194.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander WS, Viney EM, Zhang JG, et al. Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc Natl Acad Sci USA. 2006;103:16442–16447. doi: 10.1073/pnas.0607872103. [DOI] [PMC free article] [PubMed] [Google Scholar]