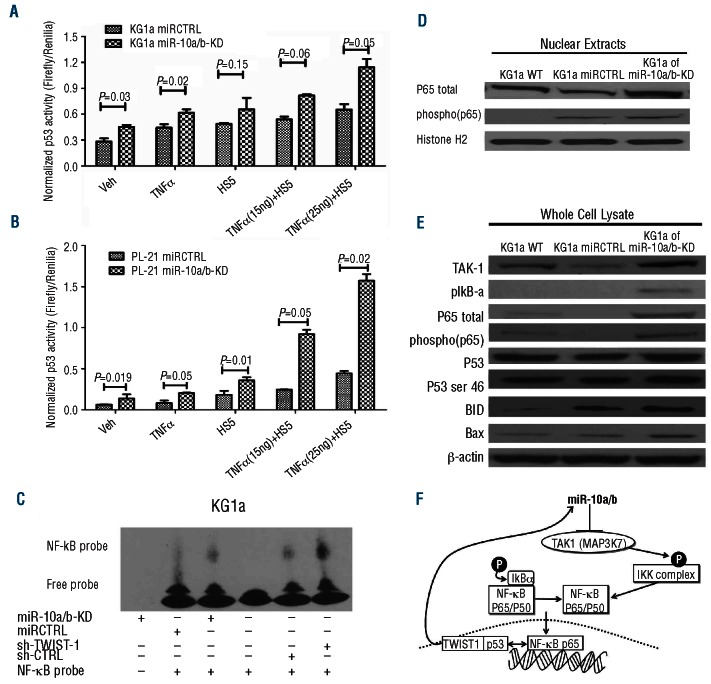
Figure 2.
TWIST-1-dependent miR10a/b expression impacts p53 activity and regulates downstream targets of NF-κB. (A) and (B) p53 transcriptional activity in KG1a miR10a/b KD KG1a miRCTRL cells, PL-21 miR10a/b KD and PL-21 miRCTRL cells; cell were cultured by themselves (none) or with the addition of TNFα (TNFα), in contact with stroma (HS5) or both (TNFα + HS5). Luciferase assays showed increased p53 transcriptional activity in KG1a (p53 mutant) and PL-21 cells (p53 wild type) with knockdown of mir10a/b both in the presence and absence of HS5 stroma cells and TNFα (15 ng/mL and 25 ng/mL) exposure, in comparison to scrambled controls (miRCTRL). (Results show the mean±SEM of 3 experiments; Student’s t-test). (C) Activation of NF- κB in KG1a miR10a/b KD, KG1a and sh-TWIST-1 as shown by Electrophoretic Mobility Shift Assay (EMSA). (D) Nuclear protein lysates from KG1a, miRCTRL and miR10a/b KD cells, separated on 4%–12% Bis-Tris gels and immunoblotted with antibodies against p65 total and phospho-p65. Histone H2 served as loading control (one of 2 similar experiments). (E) Protein lysates from KG1a wt, miRCTRL and miR10a/b KD cells, were separated on 4%–12% Bis-Tris gels and immunoblotted with antibodies against TAK-1, pIKB-α, p65 total, phosphor- p65, p53, p53-ser46, BID and Bax, respectively; β-actin served as loading control (one of 2 similar experiments). (F) Proposed working model summarizing the regulatory network involving TWIST-1, miR10a/b and TAK-1 in myeloid cell lines.