Abstract
Mucosa associated lymphoid tissue lymphoma shares certain features with multiple myeloma. In view of this and the activity of lenalidomide in various B-cell lymphomas, we have initiated a phase II study of lenalidomide in patients with mucosa associated lymphoid tissue lymphoma. Patients with histologically verified advanced stages of this lymphoma were included in the study. Treatment consisted of oral lenalidomide 25 mg Days 1–21, with a 7-day break after each cycle. A total of 18 patients were included in the trial: 5 had gastric and 13 had extragastric mucosa associated lymphoid tissue lymphoma, but 2 discontinued therapy during the first course of therapy. In the intent to treat analysis, an overall response rate of 61% was seen (11 of 18; 6 complete and 5 partial remissions). Three patients had stable disease while 2 progressed. Side effects were manageable and included neutropenia (grade III in 3 patients) as the leading hematotoxicity. After a median follow up of 20.3 months, one patient has died from lymphoma while the remaining patients are alive and relapse-free. These data suggest activity of lenalidomide monotherapy in mucosa associated lymphoid tissue lymphoma. The study protocol had been approved by the Ethical Board of the Medical University Vienna (EK-No.: 146/09), and before opening the trial, it had been registered at www.clinicaltrials.gov. (identifier: NCT00923663).
Introduction
Extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT-lymphoma) accounts for approximately 8% of all newly diagnosed lymphomas.1 The cell of origin is postulated to be a marginal zone B cell, with a close relationship to the plasma cell.2
Antibiotic eradication of Helicobacter pylori (HP) has become the mainstay of therapy for patients with gastric MALT lymphoma, while the role of antibiotics has not been unequivocally clarified in ocular adnexal MALT lymphoma. In patients with non-response to HP-eradication or non-gastric MALT lymphoma, different treatment approaches have been published, including radiation in case of localized disease, as well as a variety of systemic treatments. However, standards have still not been clearly defined.3
Lenalidomide belongs to a class of compounds termed immunomodulatory derivatives of thalidomide (IMiDs), which display both anti-angiogenic as well as immunomodulatory effects.4 Due to its high activity in multiple myeloma, a phase II study of thalidomide in MALT lymphoma was initiated, but was closed prematurely due to the absence of activity in the initial 8 patients.5 Recent studies have shown lenalidomide to be of similar if not higher efficacy in mutiple myeloma6–8 and a variety of B-cell lymphomas.9–12
In view of the close relation between multiple myeloma and MALT lymphoma, the immunological component in the genesis of this disease, as well as a probably increased activity over thalidomide, we have performed a phase II study to assess the therapeutic efficacy of lenalidomide in MALT lymphoma.
Design and Methods
Patients with histologically confirmed MALT lymphoma according to the recent WHO classification1 aged between 18 and 80 years were eligible for the trial, and all histologies were (re-)assessed by a reference hematopathologist (LM).
The study protocol had been approved by the Ethical Board of the Medical University Vienna, and before opening the trial, it had been registered at www.clinicaltrials.gov.
Patients with non-gastric MALT lymphoma or HP-negative gastric MALT lymphoma were directly eligible for inclusion, while patients with gastric MALT lymphoma and evidence of HP-infection had to have documented non-response to antibiotic therapy as judged by a minimum follow up of 12 months after successful HP-eradication. For inclusion in the trial, patients were required to have an ECOG performance status of 0–2, a life expectancy of at least three months, adequate cardiac, renal and liver function tests, and had to give their written informed consent.
Exclusion criteria were use of any investigational agent within 28 days prior to initiation of treatment with lenalidomide, history of malignancy other than squamous cell carcinoma, basal cell carcinoma of the skin or carcinoma in situ of the cervix within the last five years or major surgery, other than diagnostic surgery, within the last four weeks. In addition, patients with evidence of central nervous system (CNS) involvement, a history of uncontrolled seizures, CNS disorders or psychiatric disability judged by the investigator to be clinically significant and adversely affecting compliance to study drugs, severe peripheral polyneuropathy, clinically significant cardiac disease or myocardial infarction within the last six months, inadequate hematologic status at baseline prior to study entry, with active opportunistic infections, pregnancy, uncontrolled diabetes mellitus, pre-existing thromboembolic events or documented hypersensitivity to thalidomide were ineligible.
Treatment consisted of oral lenalidomide given at a dose of 25 mg daily for 21 days, and cycles were repeated every 28 days. As thromboembolic prophylaxis, all patients had to take 100 mg of acetylsalicylic acid daily while on study. Restaging was performed after the third cycle of therapy, and patients with at least stable disease or better were given another three courses for a maximum of six cycles. Response was assessed by radiological criteria for complete remission (CR), partial response (PR), stable disease (SD) and progressive disease (PD) in patients with non-gastric lymphoma, while patients with gastric MALT lymphoma were also assessed by applying the GELA criteria13 for histologicalal response on biopsies obtained by gastroscopy.
Results and Discussion
A total of 18 patients (10 female and 8 male) were included in the trial. Two patients discontinued therapy during the first course for personal reasons, while the remaining 16 patients underwent at least one assessment for response.
Information concerning localization of the lymphoma and patients’ clinical characteristics are shown in Table 1. Five patients had gastric MALT lymphoma, 7 ocular adnexal, 3 pulmonary, while one patient each had MALT lymphoma of the colon, the subcutis and the parotid gland, respectively. Eleven patients were therapy-naive, while the remaining patients had undergone various forms of therapy before inclusion in the trial either for progressive disease or recurrence of the lymphoma. Four patients had an underlying autoimmune disease (2 patients each with chronic autoimmune thyroiditis and Sjögren’s syndrome). Sufficient material for genetic testing was available for 14 patients, 3 had a t(11;18)(q21;q21) and one trisomy 3. Two patients discontinued treatment during the first course of therapy (one patient due to pruritus on the scalp without objective changes and the other patient for vertigo after four days of treatment), and were replaced according to protocol in order to reach the target number of 16 patients. Twelve patients were given six cycles, and 3 discontinued therapy after three courses of treatment (due to PD in one patient, for personal reasons while being in SD in another patient and due to transformation to diffuse large B-cell lymphoma in the stomach without any sign of a remaining small cell component in the third patient). Another patient with parotid lymphoma withdrew consent for further therapy after five courses due to painful parotid swelling; this returned to normal within four weeks after discontinuation of therapy. Radiological re-assessment showed ongoing SD, and the phenomenon was rated as a tumor flare. Side-effects were mainly non-hematologic, while only 3 patients had transient neutropenia WHO grade III (along with leukocytopenia in one patient); these were not complicated by infection. Non-hematologic toxicities varied widely, and 2 patients required hospitalization either due to pneumonia (in the absence of hematologic toxicities in this patient) or exanthema with desquamation. In both patients, a consecutive dose reduction to 15 mg daily did not result in recurrence of severe symptoms. In a third patient, dose reduction to 15 mg was performed for diarrhea WHO grade II after the third course of therapy, and the remaining three courses could be tolerated without any further toxicities. Interestingly, the most commonly encountered side-effect was pruritus WHO grade I/II occurring in a total of 7 patients, with 5 of these patients complaining of isolated pruritus of the scalp without dermatological changes. Table 2 shows detailed information on non-hematologic toxicities. One patient developed a biliary colic and one patient required surgery for a fracture of the femur following an accident; both events were rated as non-related to the study drug. In terms of efficacy, 11 of 18 patients (61.1%) achieved an objective response in the intent to treat analysis, including 6 patients with CR (33.3%) and 5 with PR (27.8%) as best response, while 3 patients were rated as SD (16.7%). When classified by prior therapy, 3 of 7 patients with prior systemic treatment responded, while 8 of 11 previously untreated patients also showed an objective response to therapy. A conversion to a better response from the first to the second restaging (i.e. after three and six courses, respectively) was seen in 5 patients (SD to PR and PR to CR in 2 patients each, and SD to CR in one additional patient). One patient with CR was rated as PR/responding residual disease according to GELA13 after six courses of therapy, and developed a CR nine months after the end of therapy. In addition, another patient with gastric lymphoma rated as SD after three and six cycles developed a PR/responding residual disease five months after stopping lenalidomide. Two patients had progressive disease after three courses of therapy, including one patient who showed only pure diffuse large B-cell lymphoma in the stomach as opposed to the initial MALT lymphoma. In spite of the absence of radiological progression, the patient was rated as treatment failure/PD due to the occurrence of this transformation. Both patients were switched to second-line therapy, resulting in CR after R-CHOP now ongoing for 20 months in the patient with transformation and in disease stabilization following R plus bendamustine in the other patient. In the latter, however, this stabilization lasted only four months, and the patient died from progressive lymphoma. After a median follow up of 20.3 months (range 8.4–31.2 months), the remaining 17 patients are alive without signs of lymphoma progression/recurrence (Table 1), and only one patient was lost to follow up after 11 months in SD. No second malignancies have been reported to date. Four patients had documented autoimmune diseases which remained clinically unchanged during treatment with lenalidomide.
Table 1.
Characteristics of MALT lymphoma patients receiving lenalidomide.
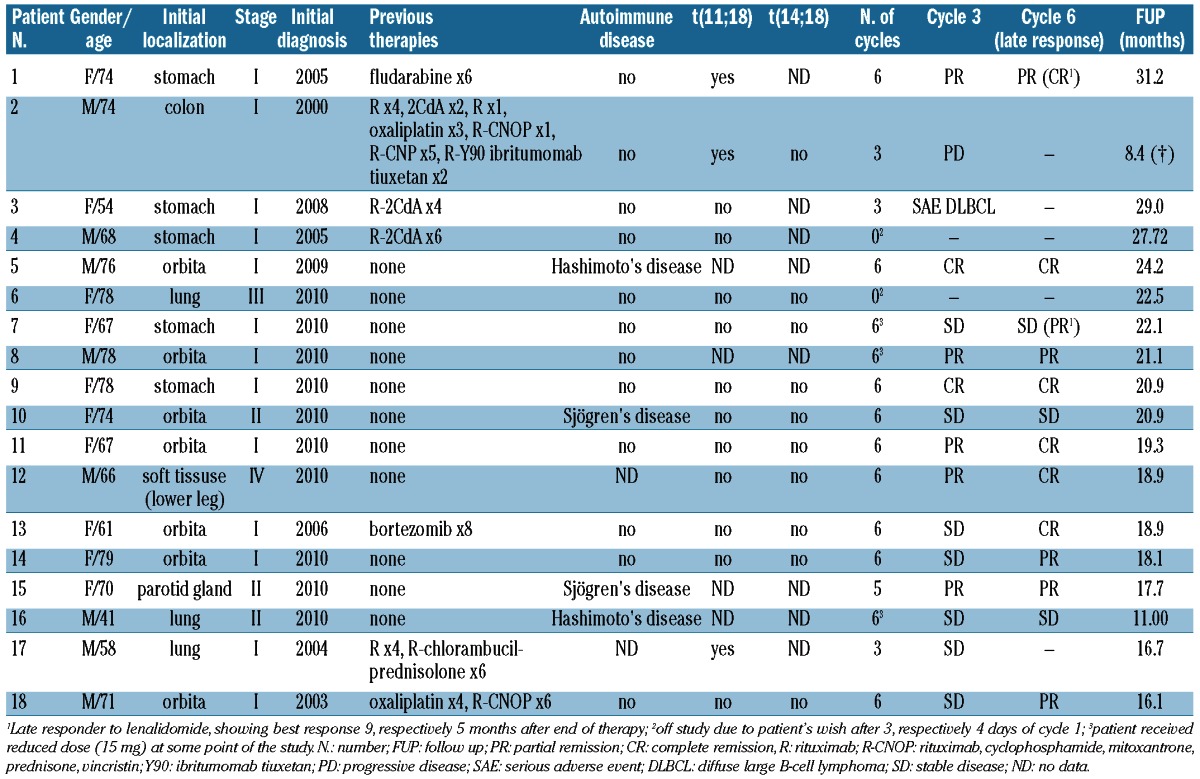
Table 2.
Characteristics of hematologic and non-hematologic toxicities

Recently, lenalidomide has been studied in patients with various histological subtypes of B-cell lymphomas, such as mantle cell lymphoma and diffuse large B-cell lymphoma, either transformed or de novo, and chronic lymphocytic leukemia.11,12,14–16 As monotherapy, in a study by Habermann et al.,11 a response rate of 53% (3 CR and 5 PR out of 15 patients) was seen in patients with mantle cell lymphoma, while in a study by Wiemik et al.,9 this was 35% in relapsed or refractory aggressive lymphomas, including 12% CR. In this latter study, it was also reported that 25% of patients with SD at initial evaluation improved with more prolonged treatment, suggesting that a more prolonged schedule of therapy might be beneficial when administering lenalidomide.9
To our knowledge, this is the first study to assess the activity of lenalidomide monotherapy in patients with MALT lymphoma indicating activity of lenalidomide at an objective response rate of 61% (11 of 18). However, only 6 patients achieved a CR (33.3%) while 5 had a PR (27.8%). In 5 patients, the results changed with continous therapy, as 2 patients with SD after the third course achieved PR, 2 PRs switched to CR, after six courses, and one patient from SD to CR. One initial PR after six cycles of lenalidomide converted to CR nine months after discontinuation of therapy, while another patient was rated as stable after six cycles achieved a PR five months later. In total, 7 of 18 patients (38.9%) benefitted from prolonged duration of therapy, suggesting that the 6-month treatment schedule as applied in our protocol might have been too short. This is in keeping with findings in other hematologic malignancies, as prolonged duration of therapy with lenalidomide has been suggested to be beneficial in terms of responses.
Two patients discontinued therapy before first assessment of response (during cycle 1) and were replaced according to protocol. In one patient, itching of the scalp without any objective dermatological findings was the reason for early discontinuation, while the other patient complained of vertigo on Day 4 of cycle 1 and refused further therapy. Out of the remaining 16 patients, 12 patients were given the planned six cycles of therapy (3 with a dose reduction to 15 mg), one patient finished five courses while 3 patients discontinued treatment after three courses (one patient due to PD, one for transformation to diffuse large B-cell lymphoma, and one for personal reasons). Hematologic side-effects were rare, and only 3 patients experienced neutropenia WHO grade III. The rate of non-hematologic toxicities, however, varied widely and the most prominent side-effect was mild itching/pruritus in 7 patients. Interestingly, the majority of patients (5 of 7) located the itching sensations on the scalp. In 4 patients, an additional exanthema on the trunk was noted which required hospitalization due to massive erythrodermia and desquamation in one case. One patient developed symptoms consistent with a tumor flare in a parotid MALT lymphoma during cycle 5. This is highly unusual, as most tumor flares in patients with lymphoid malignancies have been described early in the course of therapy.17,18 As this patient suffered from underlying Sjögren’s syndrome, one cannot rule out an interaction. In the other 3 patients with underlying ADs, however, no increased side-effects or tumor flares were documented.
Taken together, these data show that lenalidomide is able to induce objective responses in MALT-lymphoma and merits further study.
Supplementary Material
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Isaacson PG, Chott A, Nakumura S, MüllerHermelink HK, Harris NL, Swerdlow SH. Extranodal marginal cell lymphoma of mucosa-associated tissue (MALT-lymphoma). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al.WHO classification of tumours of the haematopoietic and lymphoid tissues. IARC: Lyon: 2008:214–9 [Google Scholar]
- 2.Wöhrer S, Troch M, Streubel B, Hoffmann M, Müllauer L, Chott A, et al. Pathology and clinical course of MALT lymphoma with plasmacytic differentiation. Ann Oncol. 2007;18(12):2020–4 [DOI] [PubMed] [Google Scholar]
- 3.Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60(6):747–58 [DOI] [PubMed] [Google Scholar]
- 4.Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24 (Suppl 1):S13–9 [DOI] [PubMed] [Google Scholar]
- 5.Troch M, Zielinski C, Raderer M. Absence of efficacy of thalidomide monotherapy in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Ann Oncol. 2009;20(8):1446–7 [DOI] [PubMed] [Google Scholar]
- 6.Kapoor P, Kumar S, Mandrekar SJ, Laumann KM, Dispenzieri A, Lacy MQ, et al. Efficacy of thalidomide- or lenalidomide-based therapy in proliferative multiple myeloma. Leukemia. 2011;25(7):1195–7 [DOI] [PubMed] [Google Scholar]
- 7.Gay F, Hayman SR, Lacy MQ, Buadi F, Gertz MA, Kumar S, et al. Lenalidomide plus dexamethasone versus thalidomide plus dexamethasone in newly diagnosed multiple myeloma: a comparative analysis of 411 patients. Blood. 2010;115(7):1343–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielmelli T, Bringhen S, Rrodhe S, Gay F, Cavallo F, Berruti A, et al. Previous thalidomide therapy may not affect lenalidomide response and outcome in relapse or refractory multiple myeloma patients. Eur J Cancer. 2011;47(6):814–8 [DOI] [PubMed] [Google Scholar]
- 9.Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(30):4952–7 [DOI] [PubMed] [Google Scholar]
- 10.Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(32):5404–9 [DOI] [PubMed] [Google Scholar]
- 11.Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenaliodomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–9 [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–7 [DOI] [PubMed] [Google Scholar]
- 13.Copie-Bergman C, Gaulard P, Lavergne-Slove A, Brousse N, Fléjou JF, Dordonne K, et al. Proposal for a new histological grading system for post treatment evaluation of gastric lymphoma. Gut. 2003;52(11):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novakowski GS, LaPlant B, Habermann TM, Rivera CE, Macon WR, Inwards DJ, et al. Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia. 2011;25(12):1877–81 [DOI] [PubMed] [Google Scholar]
- 15.Czuczman M, Vose JM, Witzig TE, Zinzani PL, Buckstein R, Polikoff J, et al. The differential effect of lenalidomide monotherapy in patients with relapsed or refractory transformed non-Hodgkin’s lymphoma of distinct histological origin. Br J Haematol. 2011;154(4):477–81 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in non-germinal center B-cell like than in germinal center B-cell like phenotype. Cancer. 2011;117(22):5058–66 [DOI] [PubMed] [Google Scholar]
- 17.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–9 [DOI] [PubMed] [Google Scholar]
- 18.Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and chronic lymphocytic leukemia. Blood. 2008;111(11):5291–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


