Abstract
POEMS syndrome is a rare clonal plasma cell disease. Patients with POEMS syndrome are at risk of developing pulmonary hypertension, but the data on its incidence and impact on outcome are limited. We reviewed records of 154 POEMS syndrome patients with complete duplex echocardiography data for estimation of pulmonary artery systolic pressure (sPAP) at the time of diagnosis. Forty-two (27%) of 154 patients with pulmonary hypertension (estimated sPAP ≥50mmHg) were identified. Median age was 46 years (range 31–71 years). Patients with pulmonary hypertension were more likely to have peripheral edema (P=0.04), ascites (P=0.02), pleural effusion (P=0.005), and have longer time from onset to diagnosis (P=0.004) when compared with those without pulmonary hypertension. Restrictive abnormalities and decreased diffusion capacity of carbon monoxide were observed in 83% and 96% patients with pulmonary hypertension, compared with 50% and 72% in patients without pulmonary hypertension, respectively. Reversibility of pulmonary hypertension was observed after treatment of POEMS syndrome. After median follow of 32 months, survival of patients with pulmonary hypertension was worse than those without (median overall survival 54 months vs. median not reached, P=0.021). In conclusion, pulmonary hypertension is a common feature of POEMS syndrome, and is associated with signs of extravascular volume overload. Although active treatment of POEMS syndrome can reverse pulmonary hypertension, survival of these patients is worse than those without pulmonary hypertension.
Introduction
POEMS syndrome is a multi-system disease characterized by polyneuropathy, organomegaly, endocrinopathy, M protein and skin changes. Other common associated features include extravascular volume overload, Castleman’s disease, sclerotic bone lesions, papilledema, and thrombocytosis. The pathogenesis of this rare clonal plasma cell disorder is not well understood, although a causative role for elevated serum vascular endothelial growth factor (VEGF) in this syndrome has been proposed. The median survival time after diagnosis is usually more than five years, with renal failure, infection, and cardiopulmonary failure as the most frequent causes of death.1,2 Pulmonary hypertension (PH) is thought to be an uncommon feature of POEMS syndrome. Cases of PH in patients with POEMS syndrome have been reported in the literature.3–9
However, a comprehensive review of PH in POEMS syndrome is lacking. Therefore, we sought to characterize the prevalence, clinical correlates, response to treatment, and prognostic significance of PH in a retrospective study of a population of POEMS syndrome patients.
Design and Methods
Patients
We retrospectively reviewed medical records of 167 cases with a clinical diagnosis of POEMS syndrome in Peking Union Medical College Hospital from 2001 to 2011. Of these, 154 patients with complete data of Doppler echocardiography (DE) at the time of diagnosis were identified. These patients were referred to our hospital from more than 30 centers throughout the whole country. Based on the criteria from Dispenzieri et al.,10 all patients met two major criteria (polyneuropathy and monoclonal plasma proliferative disorder) and at least one of seven minor criteria (bone lesion, Castleman’s disease, organomegaly, edema, endocrinopathy, skin changes, and papilledema). This study was approved by the institutional review board of Peking Union Medical College Hospital.
The following data were collected by chart review at the time of diagnosis: demographic information including age, gender, and time from onset to diagnosis; clinical symptoms of cough and dyspnea; and POEMS-related features of neuropathy, organomegaly (lymphadenopathy, splenomegaly, and hepatomegaly), M protein, skin changes, extravascular volume overload (edema, ascites, and pleural effusion), bone lesions, serum creatinine clearance rate (CLCr), plasma cell in bone marrow, anemia, thrombocytosis and serum VEGF level. Organomegaly and extravascular volume status were evaluated by physical examination, ultrasound or computed tomography scanning. Diagnosis of bone lesion was based on a complete X-ray survey of bone (including skull, spine, pelvis, humerus and femur) without biopsy. The percent of plasma cells was determined from bone marrow aspiration. Serum VEGF level was measured as described previously.11
Measurement of sPAP and definition of PH
Two of the authors (LJ and TZ) reviewed and analyzed DE data. DE examination was performed with duplex cardiac ultrasound system (Vivid E9 or Vivid 7, GE Medical Systems, Horton, Norway) with a 1.5–4.5 MHz phased-array transducer. Transthoracic Doppler and two-dimensional images were obtained from standard parasternal long and short axis views and apical two-chamber and four-chamber views. Left ventricular volumes were calculated from orthogonal apical views using the biplane area-length method. Ejection fraction was calculated by Simpson’s rule.12 Right ventricular dimension was measured from apical four-chamber view. Tricuspid regurgitant flow was identified by color-flow Doppler, and the peak velocity was measured by continuous-wave Doppler. Systolic pulmonary arterial pressure (sPAP) was estimated based on the modified Bernoulli equation: sPAP=4v2 (v=peak velocity of tricuspid regurgitation) + right atrial pressure (RAP).13 The RAP was calculated from the diameter of the inferior vena cava and the collapsibility index. PH was defined as sPAP of 50 mmHg or over based on recently published guidelines.14
Pulmonary function tests
The percentage of predicted FEV1, FVC, total volume capacity (TLC), and diffusion capacity of carbon monoxide (DLCO), and the value of FEV1/FVC ratio were abstracted and recorded from initial reports of pulmonary function tests at the time of diagnosis. According to guidelines described previously,15 patients with predicted TLC less than 80% or predicted FVC less than 80% but with normal FEV1/FVC ratio were considered to have restrictive abnormalities. Patients with FEV1/FVC less than 75% were defined as those with obstructive abnormalities. Decreased DLCO was defined as DLCO less than 80% of predicted levels. The initial arterial oxygen pressure and carbon dioxide pressure were also recorded.
Treatment and follow up
Treatment data were recorded from medical records. Authors tried to contact all patients and the treating physicians to obtain the follow-up data, including death and cause of death, via telephone, mail or e-mail. Patients with at least one follow-up echocardiography study after treatment were identified. PH response was defined as a decrease in sPAP to less than 50 mmHg after treatment. Overall survival was calculated as the time from diagnosis to death or to the time of last visit.
Statistical analysis
Data were analyzed with the statistical software SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test was used to analyze the difference between groups for continuous variables, and Pearson’s &x003C7;2 test or Fisher’s exact test were used to analyze differences between groups for categorical variables. Survival data were analyzed by the Kaplan-Meier method with log rank test for comparison between groups. For all analyses, a two-tailed P value of less than 0.05 was considered to be statistically significant.
Results
Clinical characteristics of patients with PH
Forty-two of 154 (27%) patients with adequate DE data were identified as those with PH. Fifty-five percent of patients were male and median age was 46 years (range 31–71 years). Polyneuropathy and M protein were observed in all patients. The median time from onset to diagnosis was 26 months (range 5-120 months). The type of M protein was restrictively derived from lambda light chain including IgA-lambda (67%), IgG-lambda (21%) and lambda alone (12%). Median sPAP was 59 mmHg (range 50–101 mmHg) and 12 patients (29%) had severe PH with sPAP of 70 mmHg or over. All patients had normal left ventricular ejection fraction above the lower limit of our echocardiography laboratory (≥55%). The median left ventricular ejection fraction was 67% (range 56-81%). The median values of right ventricular dimension and inferior vena cava dimension were 25 mm (range 21–35 mm) and 13 mm (range 10–20 mm), respectively. No hemodynamically significant valvular abnormality was identified in any of the patients while 72% of patients had pericardial effusion.
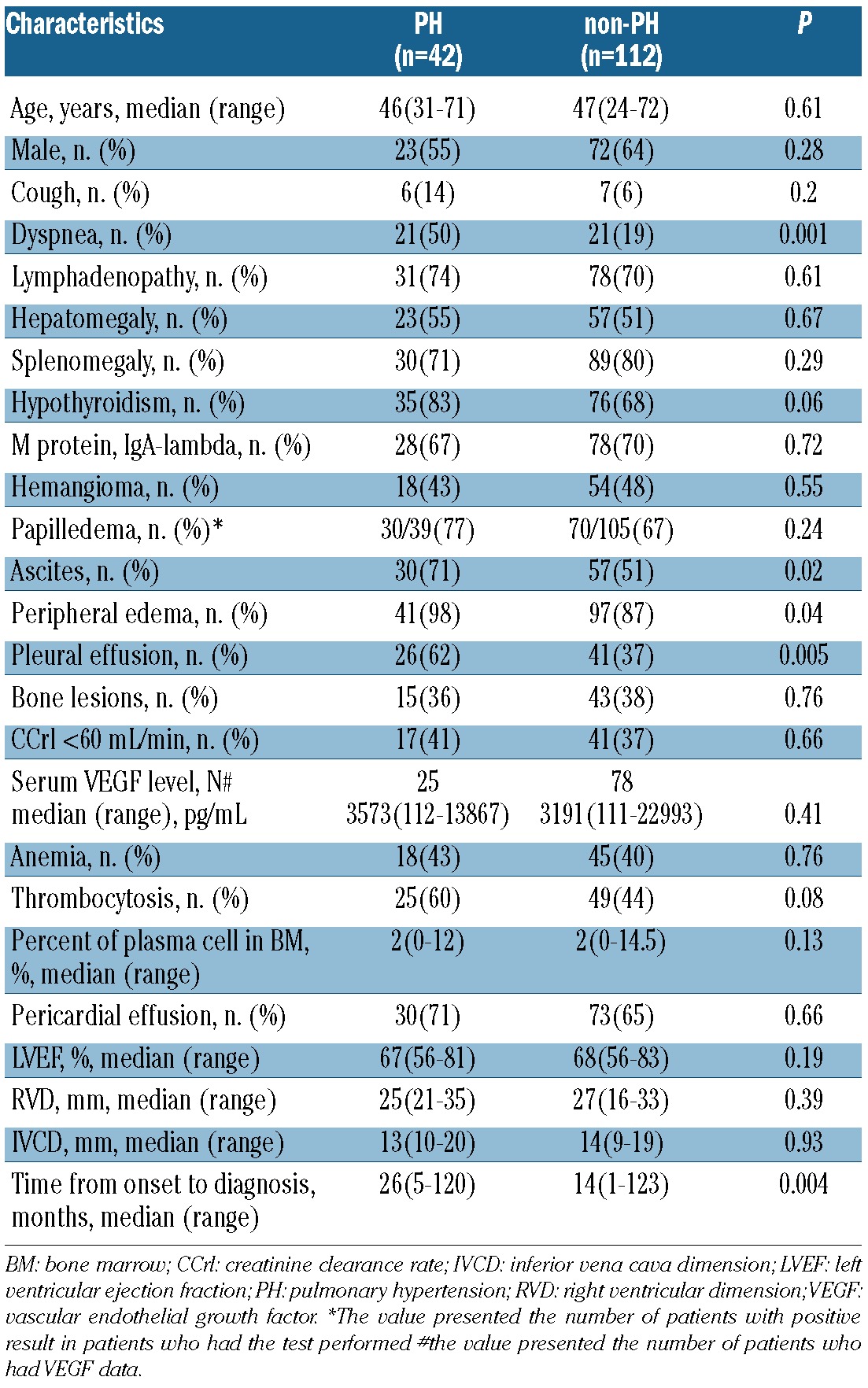
Comparison of clinical features between patients with and without PH
Table 1 shows characteristics of patients with and without PH. The median time from onset to diagnosis in the PH group was longer than that in the non-PH group (26 vs. 14 months, P=0.004). Dyspnea was reported more frequently in patients with PH (50% vs. 19%, P=0.001). Patients with PH were more likely to have ascites (71% vs. 51%, P=0.02), edema (98% vs. 87%, P=0.04), and pleural effusion (62% vs. 37%, P=0.005). There were no differences in age, gender distribution, symptom of cough, lymphadenopathy, splenomegaly, hepatomegaly, hypothyroidism, M protein type, hemangioma, papilledema, bone lesions, renal function, serum VEGF level, anemia, thrombocytosis, plasma cell percent in bone marrow, pericardial effusion, left ventricular ejection fraction, right ventricular size and inferior vena cava dimension between these two groups.
Table 1.
Comparison of clinical characteristics between patients with and without PH.
Relationship between PH and PFTs
Eighty-three of the 154 patients with DE data underwent PFTs at the time of diagnosis. Decreased DLCO was the most common PFTs abnormality, observed in 78% of patients. Restrictive lung disease was present in 59% and obstructive lung disease in 2%.
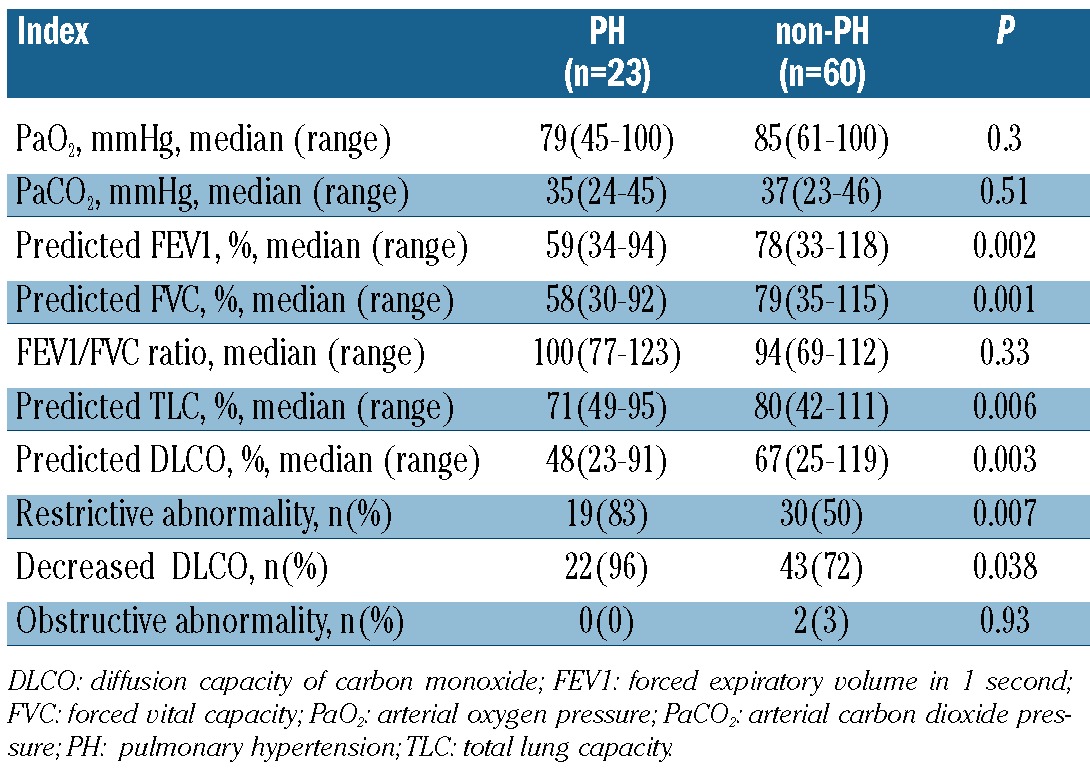
We compared the indexes of PFTs and arterial blood gas results between patients with and without PH (Table 2). Compared with patients without PH, patients with PH had significantly decreased predicted values of FEV1 (59% vs. 78%, P=0.002), FVC (58% vs. 79%, P=0.001), TLC (71% vs. 80%, P=0.006), and DLCO (48% vs. 67%, P=0.003). However, there were no significant differences in arterial oxygen pressure (79 vs. 85mmHg, P=0.30), arterial carbon dioxide pressure (35 vs. 37 mmHg, P=0.51), or FEV1/FVC ratio (100% vs. 94%, P=0.33). Therefore, the incidence of restrictive abnormality (83% vs. 50%, P=0.007) and decreased DLCO (96% vs. 72%, P=0.04) was higher in patients with PH than in those without PH.
Table 2.
The indexes of PFTs and arterial blood gas in patients with and without PH.
Treatment, PH response and survival
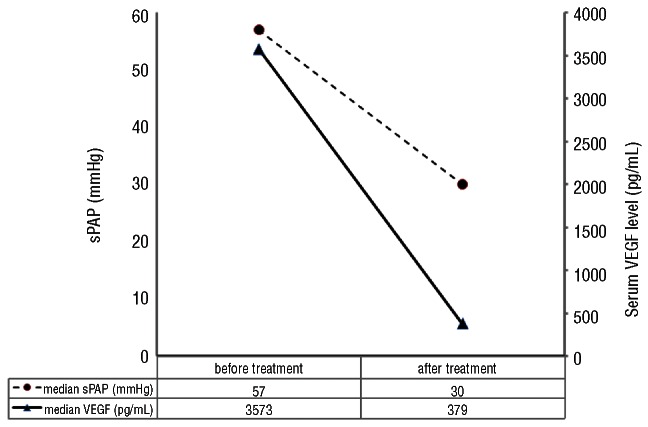
Follow-up data were obtained from 136 patients with DE data (38 patients with PH and 98 patients without PH). Patients with PH received the following treatments: melphalan and dexamethasone (MDex) (n=13), autologous stem cell transplantation (ASCT) (n=10), melphalan and prednisone (MP) (n=5), thalidomide and dexamethasone (TDex) (n=1), prednisone or dexamethasone alone (n=4), traditional Chinese medicine (TCM) (n=2), and no treatment due to early (within one month after diagnosis) death (n=3) (Table 3). Thirty-three of 38 patients under went at least one additional DE examination after treatment. The median time from treatment to the follow-up DE was 8 months (range, 3–48). PH response was observed in 88% of patients, 96% in patients treated with melphalan-based therapies including MDex, MP and ASCT and 0% in 2 patients treated with TCM. Serial serum VEGF levels were available in 23 patients with PH who had received MDex (n=13), ASCT (n=9), and TDex (n=1). The change of serum VEGF level in response to treatment paralleled the reversal of sPAP (Figure 1).
Table 3.
Treatment, PH response and survival of patients with PH.
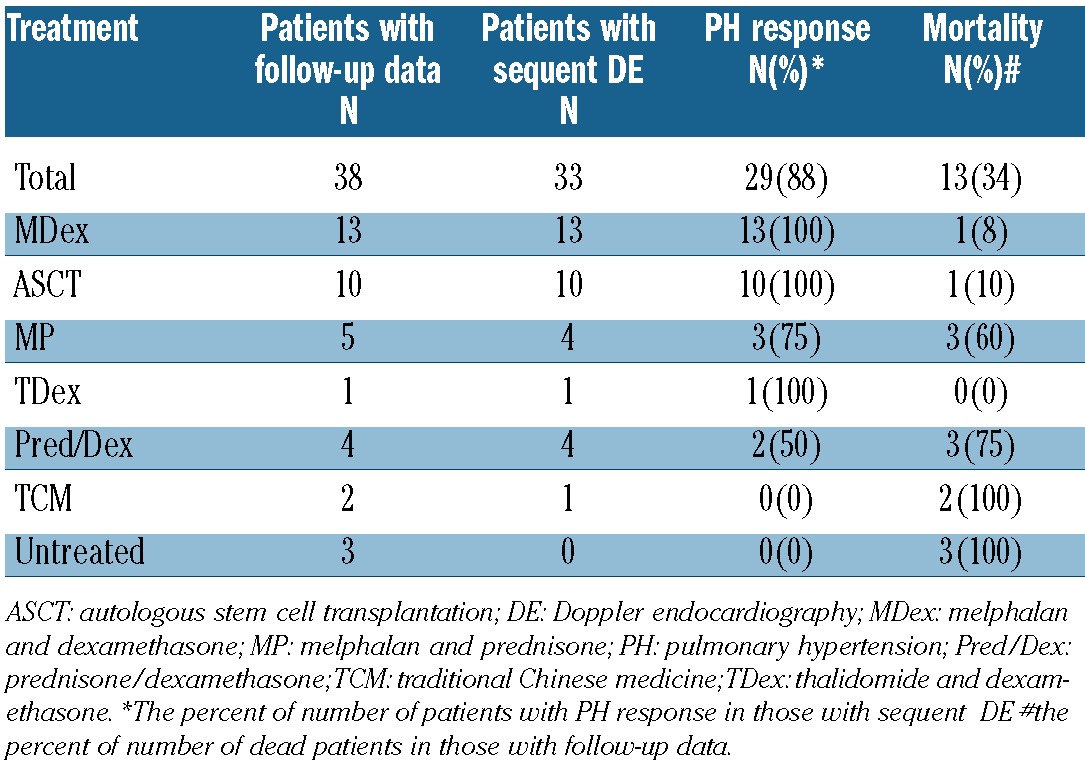
Figure 1.
sPAP and serum VEGF level in 23 patients with PH before and after treatment.
After a median follow up of 32 months, 27 of 136 patients had died. The median overall survival of all 136 patients with follow-up data was 93 months. Median overall survival had not been reached for patients without PH, while median overall survival for patients with PH was 54 months (P=0.021) (Figure 2). Thirteen (34%) patients with PH died; causes of death included progressive disease-related renal failure (n=5), cardiopulmonary failure (n=3), septic shock during ASCT (n=1), diffuse tuberculosis infection after MDex (n=1), secondary gastric cancer after MP (n=1), and unknown cause (n=2).
Figure 2.
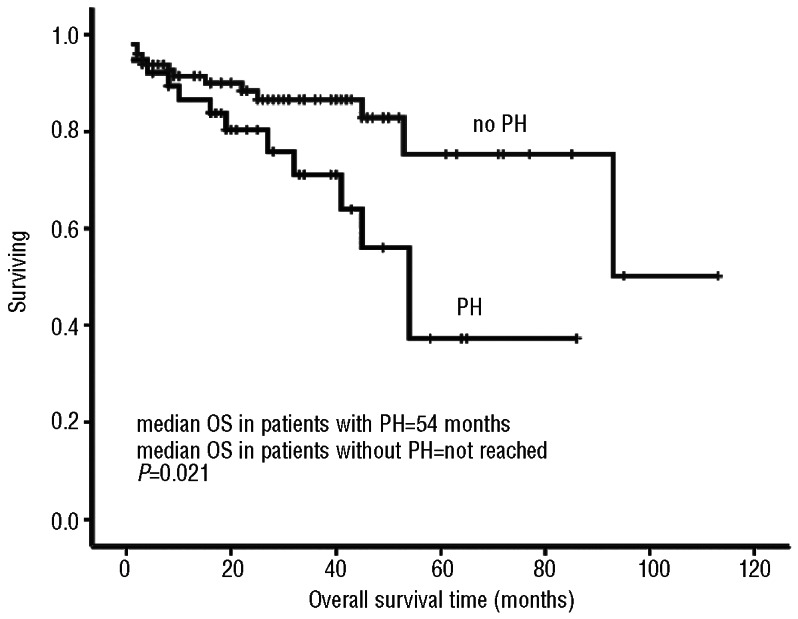
Overall survival times in patients with and without PH.
Discussion
To our knowledge, the current study is the largest series of POEMS syndrome and PH. It had been reported that PH occurs in 33–48% of patients with POEMS syndrome, depending on the definition of PH and the size of the series. In the present study, we found prevalence for PH of 27% in 154 patients, using DE for evaluation for PH, with a sPAP of 50 mmHg as the cut-off value. This cut-off value is recommended for the diagnosis of likely PH, according to recently published guidelines of the European Respiratory Society and European Society of Cardiology.14
The pathogenesis of POEMS-related PH is not clear. However, sPAP measured from DE cannot distinguish pulmonary arterial hypertension from pulmonary venous hypertension from severe left ventricular dysfunction. Additional catheterization data for evaluation of PH were lacking due to the retrospective nature of this study. Pulmonary venous hypertension (or post-capillary hypertension) is less likely in our patients. All our patients with PH had normal LVEF. Diastolic function could not be assessed due to incomplete documentation and measurements. However, severe diastolic dysfunction with pulmonary venous hypertension is most commonly observed in elderly subjects and, therefore, is not a likely cause of PH in this relatively young patient population. Cytokine-mediated proliferation of endothelial cells with resultant pulmonary vasculopathy is thought to be one of the causative mechanisms of PH in POEMS syndrome.5,16–18 VEGF, a key proinflammatory cytokine for POEMS syndrome, can cause microvascular hyperpermeability leading to extravascular volume overload, stimulate excess endothelial proliferation, activate vascular smooth cell, and induce vascular endothelial dysfunction.19–22 In our study, we found that PH was significantly associated with signs of extravascular volume overload (edema, ascites, and pleural effusion). These signs were not likely induced by PH, as there was no evidence of right ventricular failure (normal right ventricular dimension and inferior vena cava dimension). Accordingly these findings suggest that PH might be one of the downstream presentations similar to extravascular volume overload caused by a similar mechanism, including excess serum VEGF. In addition, the finding that the improvement of PH after treatment was accompanied by a reduction in serum VEGF level also suggests that these two are closely related, besides a causal relationship.
However, there was no difference in serum VEGF level between patients with and without PH in this study. This is not surprising since the absolute value of serum VEGF level is not associated with the severity of symptoms, and patients with low serum VEGF may have more severe symptoms than those with high levels of VEGF. In fact, VEGF may not be the only explanation for these symptoms based on the conflicting results of anti-VEGF antibody therapy.23,24
In our study, we found that patients with PH had longer time from onset to diagnosis than those without. Allam et al.4 also reported that 6% patients developed PH over the follow-up period. Therefore, the prevalence of PH in POEMS syndrome might be associated with the duration of disease.
PH patients reported more symptom of dyspnea than non-PH patients despite similar arterial oxygen pressure in two groups. Decreased DLCO and restrictive abnormality are common PFT abnormalities in patients with POEMS syndrome. This finding is consistent with that reported by Dispenzieri et al., although we did not record inspiratory and expiratory pressures to document the role of neuro-muscular weakness in this study. The restrictive abnormality and decreased DLCO were more common in patients with PH than in patients without. Pleural effusion, ascites and organomegaly could also contribute to these two PFT abnormalities. The potential pulmonary interstitial edema associated with extravascular volume overload might be another mechanism.
Several studies have reported that PH in POEMS syndrome is reversible after successful treatment of POEMS syndrome, including ASCT, MDex, and corticosteroids.5,6,8,9,25–27
In our study, the PH response rate was 88% in 33 patients at a median eight months after treatment. Ninety-six percent of patients who received melphalan-based therapy achieved PH response; this was higher than those who received prednisone or dexamethasone alone (50%). The reversibility of PH may partially explain the longer median survival of 54 months in our cohort when compared with a median survival of 2.8 years in a previous report of POEMS syndrome patients with symptomatic PH.28
Although a high rate of PH response to treatments was observed in this study, we found that patients with PH had worse median survival than those without (54 months vs. not reached, P=0.021). High early mortality due to aggressive disease might contribute to this shorter survival as 3 patients died within one month after diagnosis (without treatment) and 62% patients died of disease progression-related complications (renal failure, or cardiopulmonary failure). However, it is worthy of note that no one died of disease progression in patients receiving ASCT or MDex, and only 2 patients died of treatment-related infections. Therefore, active treatments including ASCT and MDex may improve prognosis of patients with POEMS syndrome and PH. Novel agents (thalidomide, lenalidomide, and bortezomib) might also be used for patients with aggressive POEMS syndrome.
Our study has several limitations due to its retrospective nature. First, our patients did not have available hemodynamic data from right heart catheterization. Right heart catheterization is the recommended test for the definitive diagnosis of pulmonary hypertension. However, risks related to this invasive procedure limits its routine use in clinical practice. DE is widely available, cost effective, and has been established as the non-invasive screening technique of choice for the detection of PH. Second, the estimated sPAP from DE is subject to variations of the signal quality of tricuspid regurgitant flow and Doppler alignment. It can overestimate or underestimate the sPAP or right ventricular systolic pressure.29 Third, there were big differences in the time to follow-up DE in 33 patients with PH to evaluate the response to the therapy and thus might have compromised an accurate estimation of the degree of response to the specific therapy. The findings of a significant reduction in the mean sPAP and concomitant significant reduction in serum VEGF level do not suggest a causal connection but these are nonetheless interesting observations that require further investigation in a prospective study.
In conclusion, PH is a common clinical feature of POEMS syndrome. All patients with POEMS syndrome should be assessed or screened for PH by DE, especially those patients with signs of extravascular volume overload. Most PH in POEMS syndrome is reversible or partially reversible after successful treatment. PH is also a negative prognostic factor for survival of POEMS syndrome patients. Active treatments including MDex, ASCT and novel agents may improve outcome of this special group of patients. The findings of this retrospective study should be validated in future prospective studies.
Supplementary Material
Acknowledgments
We thank Professor Stuart Katz who reviewed our manuscript. We thank Dr. Hui-En Li who reviewed the statistical methods and analyses.
Footnotes
Funding
This work was supported by a grant from Capital Research Fund on Clinical Application (Z111107058811019). H-YZ is supported by NIH/NHLBI grant T32HL098129.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86(7):591–601 [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. POEMS Syndrome. Blood Rev. 2007;21(6):285–99 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhou DB, Huang Z, Jiao L, Duan MH, Zhang W, et al. Clinical characteristics and long-term outcome of patients with POEMS syndrome in China. Ann Hematol. 2011;90(7):819–26 [DOI] [PubMed] [Google Scholar]
- 4.Allam JS, Kennedy CC, Aksamit TR, Dispenzieri A. Pulmonary manifestations in patients with POEMS syndrome: A retrospective review of 137 patients. Chest. 2008;133(4):969–74 [DOI] [PubMed] [Google Scholar]
- 5.Lesprit P, Godeau B, Authier FJ, Soubrier M, Zuber M, Larroche C, et al. Pulmonary hypertension in POEMS syndrome: A new feature mediated by cytokines. Am J Respiratory Crit Care Med. 1998;157(3 Pt1):907–11 [DOI] [PubMed] [Google Scholar]
- 6.Paciocco G, Bossone E, Erba H, Rubenfire M. Reversible pulmonary hypertension in POEMS syndrome: another etiology of triggered pulmonary vasculopathy? Can J Cardiol. 2000;16(8):1007–12 [PubMed] [Google Scholar]
- 7.Okura H, Gohma I, Hatta K, Imanaka T. Thiamine deficiency and pulmonary hypertension in Crow-Fukase syndrome. Intern Med. 1995;34(7):674–5 [DOI] [PubMed] [Google Scholar]
- 8.Jouve P, Humbert M, Chauveheid MP, Jais X, Papo T. POEMS syndrome-related pulmonary hypertension is steroid responsive. Respir Med. 2007;101(2):353–5 [DOI] [PubMed] [Google Scholar]
- 9.Ribadeau-Dumas S, Tillie-Leblond I, Rose C, Saulnier F, Wemeau JL, Hatron PY, et al. Pulmonary hypertension associated with POEMS syndrome. Eur Respir J. 1996;9(8):1760–2 [DOI] [PubMed] [Google Scholar]
- 10.Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(17):2496–506 [DOI] [PubMed] [Google Scholar]
- 11.Hashiguchi T, Arimura K, Matsumuro K, Otsuka R, Watanabe O, Jonosono M, et al. Highly concentrated vascular endothelial growth factor in platelets in Crow-Fukase syndrome. Muscle Nerve. 2000;23(7):1051–6 [DOI] [PubMed] [Google Scholar]
- 12.Douglas PS, Garcia MJ, Haines DE, Lai WW, Manning WJ, Patel AR, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI /SCCM/SCCT/SCMR 2011 Appropriate use criteria for echocardiography. J Am Soc Echocardiogr. 2011;24(3):229–6721338862 [Google Scholar]
- 13.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–62 [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. The guidelines for diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34(6):1219–63 [DOI] [PubMed] [Google Scholar]
- 15.Amecian Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144(5):1202–8 [DOI] [PubMed] [Google Scholar]
- 16.Niimi H, Arimura K, Jonosono M, Hashiguchi T, Kawabata M, Osame M, et al. VEGF is causative for pulmonary hypertension in a patient with Crow-Fukase (POEMS) syndrome. Intern Med. 2000;39(12):1101–4 [DOI] [PubMed] [Google Scholar]
- 17.Helmersen DS, Ford GT, Viner SM, Auger WR. POEMS syndrome: a clue to understanding primary pulmonary hypertension? A review of current insights into the pathogenesis of primary pulmonary hypertension. Can J Cardiol. 2000;16(8):975–81 [PubMed] [Google Scholar]
- 18.Feinberg L, Temple D, de Marchena E, Patarca R, Mitrani A. Soluble immune mediators in POEMS syndrome with pulmonary hypertension: case report and review. Crit Rev Oncog. 1999;10(4):293–302 [PubMed] [Google Scholar]
- 19.Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe O, Maruyama I, Arimura K, Kitajima I, Arimura H, Hanatani M, et al. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve. 1998;21(11):1390–7 [DOI] [PubMed] [Google Scholar]
- 21.Soubrier M, Sauron C, Souweine B, Larroche C, Wechsler B, Guillevin L, et al. Growth factors and proinflammatory cytokines in the renal involvement of POEMS syndrome. Am J Kidney Dis. 1999;34(4):633–8 [DOI] [PubMed] [Google Scholar]
- 22.Scarlato M, Previtali SC, Carpo M, Pareyson D, Briani C, Del Bo R, et al. Polyneuropathy in POEMS syndrome: Role of angiogenic factors in the pathogenesis. Brain. 2005;128(Pt8):1911–20 [DOI] [PubMed] [Google Scholar]
- 23.Kanai K, Kuwabara S, Misawa S, Hattori T. Failure of treatment with anti-VEGF monoclonal antibody for long-standing POEMS syndrome. Intern Med. 2007;46(6):311–3 [DOI] [PubMed] [Google Scholar]
- 24.Samaras P, Bauer S, Stenner-Liewen F, Steiner R, Zweifel M, Renner C, et al. Treatment of POEMS syndrome with beva-cizumab. Haematologica 2007;92(10):1438–9 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zhang W, Jiao L, Duan MH, Guan HZ, Zhu WG, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome. Blood. 2011;117(24):6445–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120(1):56–62 [DOI] [PubMed] [Google Scholar]
- 27.Rached S, Athanazio RA, Dias SA, Jr, Jardim C, Souza R. Systemic corticosteroids as first-line treatment in pulmonary hypertension associated with POEMS syndrome. J Bras Pneumol. 2009;35(8):804–8 [DOI] [PubMed] [Google Scholar]
- 28.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–9 [DOI] [PubMed] [Google Scholar]
- 29.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.