Fig. 1.
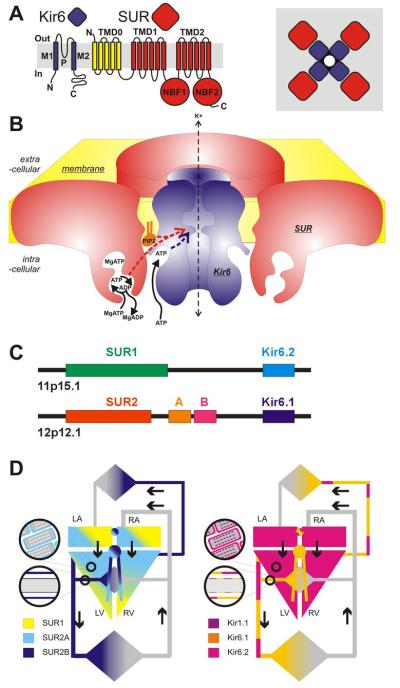
Cardiovascular KATP channels (A) Kir6 subunits generate the channel pore, SUR subunits serve the regulatory role, each channel being a functional octamer of 4 Kir6 subunits and 4 SUR subunits. (B) The metabolically controlled gate of the channel is located at the cytoplasmic end of the inner cavity. ATP binds to Kir6 subunits and this provides the energetic push to channel closure. MgATP binds to the ATP-binding sites (ABSs) formed at the NBF1-NBF2 interface on SUR subunits. ATP hydrolysis results in a conformational ‘activated’ state that is transduced to ‘over-ride’ ATP inhibition. The ‘activated state’ persists through ADP dissociation, and can be maintained by ADP rebinding. In addition, PIP2 interaction at a site near the ATP inhibitory site also provides an energetic pull to open channels, and sulfonylureas (SU) or K channel openers (KCO), interacting with the SUR subunit within the membrane, respectively cause channel closure or opening. (C) Human KATP gene structure. ABCC8 (SUR1) and KCNJ11 (Kir6.2) are immediately adjacent on chromosome 11p, whereas ABCC9 (SUR2) and KCNJ8 (Kir6.1) are immediately adjacent on chromosome 12. (D) KATP channel subunit distribution in the cardiovascular system.