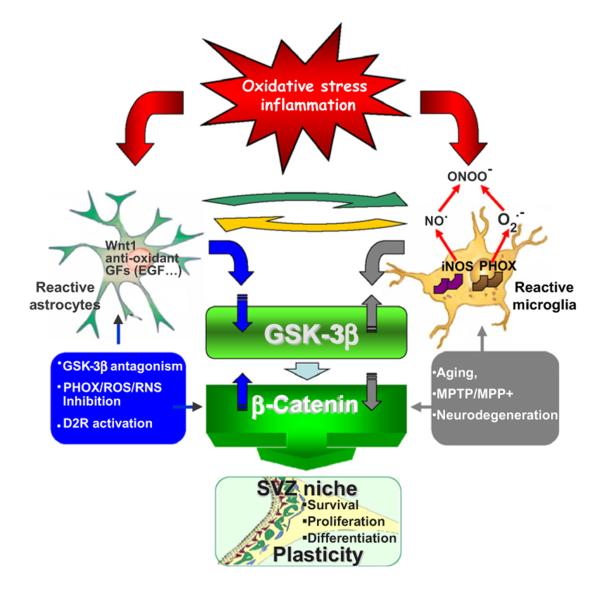
Fig. 4.
Crosstalk between inflammatory and Wnt/β-catenin signaling pathways in MPTP-induced SVZ plasticity. This is a simplified scheme summarizing MPTP-induced neuroinflammation and SVZ plasticity via modulation of Wnt/β-catenin signaling. During the early degeneration phase of MPTP toxicity, hyper-activated microglia contributes to the impairment of SVZ neurogenesis at different levels. By increasing oxidative and nitrosative stress and in synergy with MPTP/MPP+ direct toxicity, microglia-derived mediators [plasma membrane NADPH oxidase (PHOX)-derived reactive oxygen species (ROS) and iNOS-derived nitic oxide (NO) and peroxynitrite] may act as a molecular switch for cell signaling pathways that are critically involved in the physiological control of NPC homeostasis, with harmful consequences for astrocyte and NPC physiology, at least in part through GSK-3β activation, followed by phosphorylation and consequent degradation of β-catenin. By contrast, pharmacological mitigation of inflammation and oxidative stress with Apo, L-Nil or HCT1026 upregulate β-catenin and successfully rescue NPC proliferation and neuroblast formation, a process associated with striatal DAergic neuroprotection, with further positive modulation of SVZ proliferation via DA subtype 2 receptor (D2R)-activated mechanisms. The role of astrocyte–microglia interactions in the plasticity of the SVZ response to MPTP is exemplified by the astrocyte’s ability to overcome microglial inhibitory effects, also via crosstalk with Wnt/β-catenin signaling. EGF, epidermal growth factor; GF, growth factor; RNS, reactive nitrogen species. From L’Episcopo et al. with permission (2012a,b).