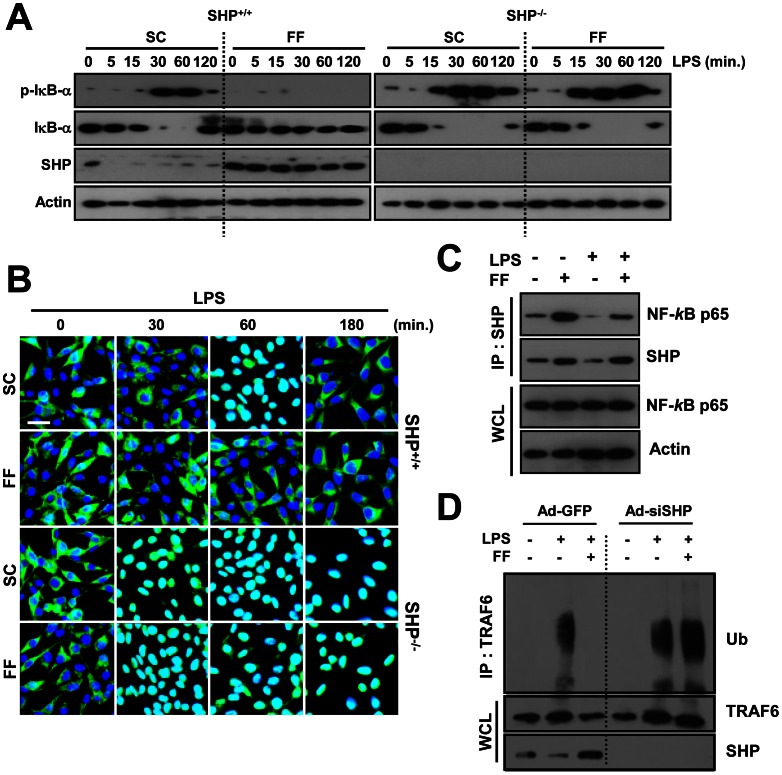
Figure 3. Fenofibrate-induced SHP regulates TLR4 signaling through interaction with NF-κB p65 and modulation of TRAF6 ubiquitination.
(A, B, and D) BMDMs from Shp +/+ and Shp −/− mice were treated with or without fenofibrate (50 µM) for 4 h, and then stimulated with LPS (100 ng/mL) for indicated times. (A) Immunoblotting (IB) was performed to determine protein expression of phosphorylated and total forms of IκB-α and SHP. Actin was used as a loading control. (B) Immunofluorescence analyses of NF-κB p65 nuclear translocation. Cells were fixed and stained with anti-NF-κB p65 (green); nuclei were counterstained with DAPI (blue). Scale bar, 20 µm. (C) RAW264.7 cells were treated with or without fenofibrate (50 µM) for 4 h, followed by LPS stimulation (100 ng/mL, 30 min). Cells were then subjected to immunoprecipitation (IP) with anti-SHP. Protein interactions between SHP and NF-κB p65 were analyzed by IB with anti-NF-κB p65. Whole cell lysates (WCL; input control) were detected by immunoblotting with anti-NF-κB p65 and anti-Actin. (D) Cell lysates were subjected to IP with anti-TRAF6 and the polyubiquitination of TRAF6 was analyzed by IB with anti-ubiquitin (Ub). WCL were detected by IB with anti-TRAF6 or anti-SHP as loading controls. The data are representative of at least three independent experiments with similar results. SC, solvent control (0.1% DMSO); FF, fenofibrate.