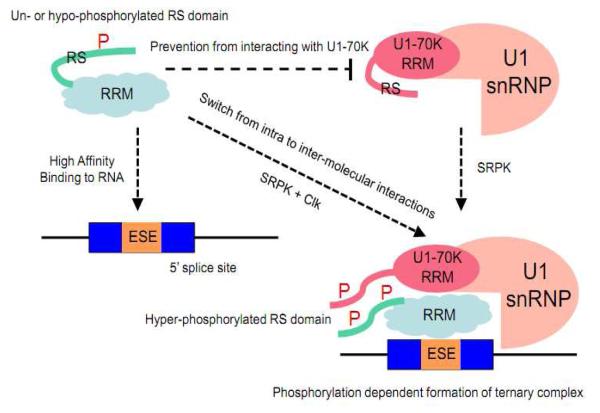
Figure 2.
Phosphorylation regulation of SR proteins in spliceosome assembly. The RS domains of both the SR protein SRSF1 and the SR-related protein U1-70K can engage in intra- and inter-molecular interactions. In un- or hypo-phosphorylated state, the RS domain is engaged in an intra-molecular interaction with one interface of the SRSF1 RRM domain, which the other interface binds RNA with high affinity. However this prevents the SRSF1 from interacting with the U1 70K protein. Hyper-phosphorylation of the RS domain of SRSF1 by SRPK1 plus Clk is proposed to switch this intra-molecular interactions to inter-molecule interactions through protein-protein interactions via both the RS domains and RRMs between SRSF1 and U1-70K, allowing the formation of the ternary complex containing the SR protein, U1 snRNP, and the RNA containing a functional 5′ splice site.