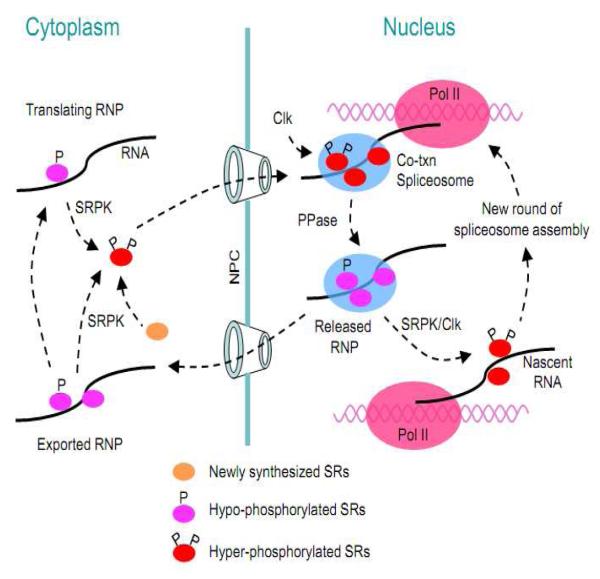
Figure 3.
Phosphorylation regulation of intercellular trafficking of SR proteins. SR proteins are initially phosphorylated by the SRPK family of kinases, which promotes nuclear import of SR proteins. After entering the nucleus, SR proteins may be further phosphorylated by the Clk family of kinases. Hyper-phosphorylated SR proteins are recruited to nascent pre-mRNA for co-transcriptional splicing. The splicing reaction requires dephosphorylation of SR proteins within the spliceosome. SR proteins associated with mRNA appear to have two alternative recycling pathways. One is through re-phosphorylation, which may release the SR protein from spliced mRNA, allowing them to recycle within the nucleus. Alternatively, hypo-phosphorylated SR proteins on spliced mRNA may be exported out of the nucleus, which functions to enhance mRNA export and stimulate translation in the cytoplasm. Re-phosphorylation in the cytoplasm may facilitate the release of the SR proteins from spliced mRNA and then promote their re-import back to the nucleus.