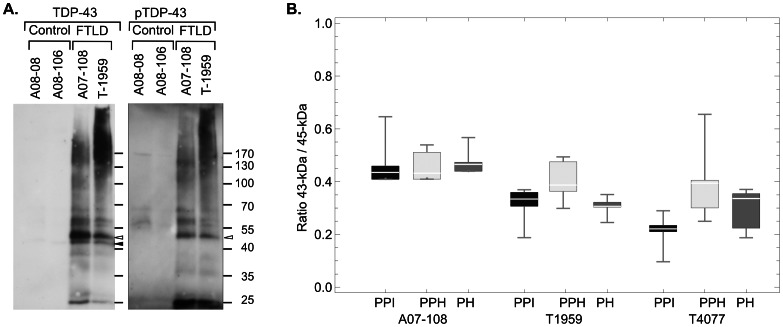
Figure 6. The 43-kDa TDP-43 band is not phosphorylated at S409/410 and not derived from 45-kDa band during processing.
(A) Immunoblot prepared exactly as those in Fig. 5A, then stripped of bound antibody and re-probed with an antibody against pS409/410 TDP-43. Phosphorylated species correspond to most of the FTLD-specific bands seen in A, including the approximately 45 kDa band (open arrowhead), but the 43-kDa TDP-43 band (closed arrowhead) is not phosphorylated at the S 409/410 site. (B) Brain homogenates were subject to conditions designed to minimize or maximize phosphatase activity (see Methods), then proteins were concentrated from the aggregate region. The quantity of the 45-kDa and 43-kDa bands were determined as described in the methods, and the ratio of the 43-kDa to 45-kDa band calculated. The box-whisker plots depict the results (range, 25–75 percentile, median) of at least 5 measurements of the ratio. The figure depicts the ratio from homogenates of 3 FTLD cases either prepared with both protease and phosphatase inhibitor stored on ice (PPI), prepared with both inhibitors and heated (PPH), or prepared with only protease inhibitor and heated (PH). An increase in the ratio of the 43-kDa to 45-kDa species in the PH condition, relative to either the PPI or PPH condition would suggest that significant endogenous phosphatase activity present in sample processing might generate the 43-kDa species from the phosphorylated 45-kDa species. No consistent pattern is seen.