Abstract
Increasing reports support that air pollution causes neuroinflammation and is linked to central nervous system (CNS) disease/damage. Diesel exhaust particles (DEP) are a major component of urban air pollution, which has been linked to microglial activation and Parkinson’s disease-like pathology. To begin to address how DEP may exert CNS effects, microglia and neuron-glia cultures were treated with either nanometer-sized DEP (<0.22 µM; 50µg/mL), ultrafine carbon black (ufCB, 50µg/ml), or DEP extracts (eDEP; from 50 µg/ml DEP) and the effect of microglial activation and dopaminergic (DA) neuron function was assessed. All three treatments showed enhanced amoeboid microglia morphology, increased H2O2 production, and decreased DA uptake. Mechanistic inquiry revealed that the scavenger receptor inhibitor fucoidan blocked DEP internalization in microglia, but failed to alter DEP-induced H2O2 production in microglia. However, pretreatment with the MAC1/CD11b inhibitor antibody blocked microglial H2O2 production in response to DEP. MAC1−/− mesencephalic neuron-glia cultures were protected from DEP-induced loss of DA neuron function, as measured by DA uptake. These findings support that DEP may activate microglia through multiple mechanisms, where scavenger receptors regulate internalization of DEP and the MAC1 receptor is mandatory for both DEP-induced microglial H2O2 production and loss of DA neuron function.
Keywords: Air pollution, brain, microglia, inflammation-mediated neurodegeneration, oxidative stress, neuroinflammation
INTRODUCTION
Accumulating evidence links air pollution exposure to central nervous system (CNS) pathology and disease (Block & Calderon-Garciduenas 2009, Guxens & Sunyer 2012). Epidemiology studies have shown that exposure to high levels of air pollution is associated with a deficit in neuropsychological development in children (Vrijheid et al. 2012, Guxens et al. 2012), cognitive decline in the elderly (Calderon-Garciduenas et al. 2008a, Weuve et al. 2012, Power et al. 2011, Ranft et al. 2009, Chen & Schwartz 2009, Suglia et al. 2008), behavioral deficits (Wang et al. 2009), autism (Volk et al. 2011), and an elevated stroke risk (Donnan et al. 1989, Henrotin et al. 2007, Villeneuve et al. 2006). Human studies have also revealed that individuals living in highly polluted cities show Alzheimer’s disease (AD)-like and Parkinson’s disease (PD)-like pathology, when compared to individuals living in cities with less pollution (Calderon-Garciduenas et al. 2004, Calderon-Garciduenas et al. 2010, Morales et al. 2009, Calderon-Garciduenas et al. 2012, Block & Calderon-Garciduenas 2009). More specifically, high levels of air pollution were associated with elevated markers of neurodegenerative disease in humans, including tau phosphorylation, diffuse β amyloid plaque deposition, and α synuclein aggregation (Calderon-Garciduenas et al. 2004, Calderon-Garciduenas et al. 2010, Morales et al. 2009, Calderon-Garciduenas et al. 2012). Human reports also reveal that air pollution causes oxidative stress, neuroinflammation, and microglial activation in the brain (Calderon-Garciduenas et al. 2008b). Consistent with human reports, animal studies have found that exposure to air pollution causes lipid peroxidation (Zanchi et al. 2010), DNA damage (Calderon-Garciduenas et al. 2003), protein nitration (Levesque et al. 2011b), elevated cytokines (Gerlofs-Nijland et al. 2010, Levesque et al. 2011b, Cassee et al. 2012, Bos et al. 2012), chemokine increases (Levesque et al. 2011b), aggregated α synuclein (Levesque et al. 2011a), increased expression of Aβ-42 in the brain (Levesque et al. 2011a), and activation of microglia (Levesque et al. 2011b, Morgan et al. 2011, Bolton et al. 2012). However, the underlying mechanisms responsible for how air pollution may cause neuroinflammation, impact neuropathology, and lead to CNS disease are largely unknown.
Diesel Exhaust (DE) has received significant attention as a human health concern in both ambient and occupational exposure conditions (Pronk et al. 2009, Hesterberg et al. 2010). DE is a major component of pollution near roadways and urban pollution (Hesterberg et al. 2010, Ma & Ma 2002), where several studies have documented the CNS effects of DE. For example, acute DE exposure has been shown to affect electroencephalogram parameters in adult human subjects (Cruts et al. 2008). Animal research also points to the prenatal period as a critical period of vulnerability, as maternal DE exposure has been shown to decrease brain DA levels and cause motor deficits in offspring (Suzuki et al. 2010, Yokota et al. 2009). Mice exposed to nanoparticle-enriched DE show elevated neuroinflammation and performance deficits in hippocampal-dependent spatial learning and memory tasks (Win-Shwe et al. 2011). Short term studies (up to 1-month exposure) show pro-inflammatory factors, such as TNFα, in the adult brain with DE exposure, using month-long inhalation models (Gerlofs-Nijland et al. 2010, Levesque et al. 2011b, Cassee et al. 2012), intratracheal administration directly into the lung (Levesque et al. 2011b), and a 2 hr-long exposure by nose-only inhalation (van Berlo et al. 2010). DE exposure also causes elevated neuroinflammation with subchronic (6 month) exposure in certain vulnerable brain regions (Levesque et al. 2011b). In fact, we have previously shown that DE elevates α synuclein levels in the midbrain, indicating that DE may impinge on PD pathology. Thus, while there are clear CNS effects with DE exposure, the underlying mechanisms are poorly understood.
At present, there are several hypotheses regarding how air pollution affects the brain. It has been proposed that soluble peripheral signals in the blood (e.g. circulating cytokines or modified lipids and proteins)(Levesque et al. 2011b), neuronal signals from the periphery, translocation of the particle components of air pollution (particulate matter, PM) to the brain(Gillespie et al. 2011), and the transfer of the chemical constituents adsorbed on the PM (e.g. polyaromatic hydrocarbons)(Cordier et al. 2004) to the brain may all regulate how air pollution cases neuroinflammation and neuropathology (Block & Calderon-Garciduenas 2009, Lucchini et al. 2012, Tonelli & Postolache 2010). While it is likely that these pathways interact to contribute to CNS health effects, postmortem sampling has identified PM in the human brain (Calderon-Garciduenas et al. 2008b), emphasizing the importance of understanding how PM and its adsorbed chemical compounds affect cells in the brain.
Microglia are the resident innate immune cell in the brain and are activated in response to diverse stimuli, including air pollution (Block & Calderon-Garciduenas 2009). Microglia have been implicated in the progressive nature of diverse neurodegenerative diseases, including PD (Block et al. 2007, Cunningham 2013, Tansey & Goldberg 2010, Kraft & Harry 2011, Schwab & McGeer 2008). Consistent with human studies (Calderon-Garciduenas et al. 2008b), we and others have demonstrated in rodent models that air pollution activates microglia (Morgan et al. 2011, Levesque et al. 2011b). Our previous work also indicates that measures of neuroinflammation in response to DE exposure in vivo are the highest in brain regions with the highest levels of the IBA-1 microglial marker, such as the midbrain, which houses the substantia nigra that is damaged in PD (Levesque et al. 2011b). We have also shown that nanometer-sized DE particles (which are components of air pollution believed to reach the brain) activate microglia in vitro, which is then neurotoxic through the production of reactive oxygen species (Block et al. 2004). Recently, we demonstrated in vitro that low concentrations of DEP amplify the microglial response to pro-inflammatory stimuli (Levesque et al. 2011b). Thus, evidence supports that DE may be a common, chronic source of microglial activation in the environment. At present, how DE causes neuroinflammation, microglial activation, and neuropathology is unknown.
The current study begins to address these issues by focusing on how DEP may be activating microglial cells to impair DA neuron function. Here, using cell lines and primary cultures we test the ability of: 1) components of DEP (ultrafine carbon particles and diesel exhaust extracts) and 2) pattern recognition receptors (MAC1 and scavenger receptors) in microglial activation and loss of DA neuron function.
MATERIALS AND METHODS
Reagents
Standard reference material (SRM) 2975 Diesel Particulate Matter (Industrial Fork Lift) and SRM 1975 Diesel Particulate Matter Extract (from SRM 2975) was purchased from the National Institute for Standards and Technology (Gaithersburg, MD). Ultrafine carbon black (ufCB, Printex 90) was a kind gift from the Degussa Corporation (Parsippany, NJ). Lipopolysaccharide (strain O111:B4) was purchased from Calbiochem (Gibbstown, NJ). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA). [3H] Dopamine (DA, 28 Ci/mmol) was purchased from NEN Life Science (Boston, MA). The polyclonal antibody against the IBA-1 microglial marker was purchased from Wako (Richmond, VA). The Biotinylated goat anti-rabbit secondary antibody was purchased from Vector Laboratories (Burlingame, CA). The monoclonal MAC1/CD11b inhibitor antibody was purchased from Lifespan Biosciences (Seattle, WA) and the normal mouse isotype IgG control antibody was purchased from Medical and Biological Research Laboratories (Woburn, MA). All other reagents were procured from Sigma Aldrich Chemical Co. (St. Louis, MO).
Animals
Timed-pregnant (gestational day 14) adult female Fisher 344 rats were purchased from Charles River Laboratories (Raleigh, NC). Eight-week-old (25–30g) male and female B6.129S4-Itgamtm1Myd/J (MAC1−/−) and C57BL/6J (MAC1+/+) were purchased from Jackson Labs (Bar Harbor Maine). The MAC1−/− mice were the result of directed mutation using a targeting vector containing neomycin resistance and herpes simplex virus thymidine kinase genes to disrupt a region of the Itgam gene encoding the translational initiation codon and 15 amino acids of the signal peptide. As such, macrophages from MAC1−/− mice are deficient at spreading, phagocytizing complement-opsonized particles, and exhibit an impaired oxidative burst. The MAC1−/− mutation is maintained in the C57BL/6J background, thus the C57BL/6J (MAC1+/+) mice were used as control animals. Breeding of the mice was designed to achieve accurate timed-pregnancy ± 0.5 days. Housing, breeding and experimental use of the animals were performed in strict accordance with the National Institutes of Health guidelines. All animal research was approved by the Virginia Commonwealth University IACUC committee.
DEP, ufCB, and eDEP preparation
Nanometer-sized DEP were used as a model of ultra-fine particulate matter and were prepared according to our previous studies (Block et al. 2004), as this is the approximate size of PM translocated to the brain and the PM fraction with the greatest neurotoxic effects (Gillespie et al. 2011). While the precise amount of particulate matter reaching the brain is unknown, current studies demonstrate that 0.01 – 0.001 % of inhaled nanometer-sized iridium and carbon particulate remain in the brain at 24 hr after exposure (Kreyling et al. 2009). Based on in vivo models of near road and occupational exposure (DEP 0.5 mg/m3, 2 mg/m3, and 20mg/kg), the in vitro concentrations of nanometer-sized particles (50–100 µg/ml) fall within the current estimates of what may reach the brain, as we have previously published (Levesque et al. 2011b). Briefly, 2 mg of DEP (DEP, NIST, SRM 2975) were added to 40 ml of treatment media and vortexed (<20s), followed by sonication for 15 min. MEM containing 2% FBS, 2% HS, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 U/mL penicillin, and 50 µg/mL streptomycin was used for neuron-glia culture treatment media. DMEM containing 2% FBS, 50 U/mL penicillin and 50 µg/mL streptomycin was used for HAPI cell treatment media. The DEP suspension was then filtered through a 0.22µm filter (Millipore, Billerica), immediately diluted to appropriate concentrations, and immediately added to the culture. The precise number of nanometer particles present is not determined. The chemical and particle characteristics of the DEP sample are readily available at the National Institute of Standards and Technology website: (https://srmors.nist.gov/view_detail.cfm?srm=2975).
ufCB (Printex 90) was used to investigate the effects of only a carbon particle without the compounds adsorbed on the surface of the DEP particle and was a kind gift from the Degussa Corporation (Parsippany, NJ, USA). ufCB was prepared in the same manner as DEP above. The average size of Printex 90 is approximately 0.17–0.19 µM (Stoeger et al. 2006), where they later appear as aggregates of various sizes in culture medium after treatment (Ovrevik et al. 2009). Aggregation was not measured in the current study.
Finally, DEP extracts (SRM 1975) were used to discern the effects of the chemical compounds adsorbed on the surface of carbon DEP particle. Extracts were prepared at NIST with 5.6 kg of DEP from an industrial fork lift (SRM 2975) using dichloromethane. The DEP extract was concentrated by evaporation under nitrogen to a final volume of 8L and distributed in 1.2 mL ampoules, representing the amount of adsorbed compounds on 700g/mL of DEP. The DEP extract was diluted approximately 1:10,000,000 to investigate the effects of the adsorbed compounds from 50µg/ml DEP. Several compounds are adsorbed on the surface of DEP, including several polyaromatic hydrocarbons that are known to be toxic to neurons (Ma & Ma 2002). The chemical composition of the DEP extracts is readily available online: (https://www-s.nist.gov/srmors/view_detail.cfm?srm=1975).
The endotoxin level in DEP (SRM 2975), ufCB, and DEP extracts (SRM 1975) was quantified using a commercially available kit (GenScript, Piscataway, NJ) and negligible amounts were found: ufCB<0.0001, eDEP<0.0001, and DEP = 0.098 EU.
Mesencephalic neuron-glia cultures
Rat and mouse ventral mesencephalic neuron-glia cultures were prepared using a previously described protocol (Liu et al. 2001). Briefly, midbrain tissues were dissected from day 14 Fisher 344 rat or mouse embryos. Cells were dissociated via gentle mechanical trituration in minimum essential medium (MEM) and immediately seeded (5×105/well) in poly D-lysine (20µg/ml) pre-coated 24-well plates. Cells were seeded in maintenance media and exposed to the treatment media, as described previously (Liu et al. 2001). Three days after seeding, the cells were replenished with 500 µL of fresh maintenance media. Cultures were treated 7 days after seeding.
Cell lines
The highly aggressive proliferating immortalized (HAPI) rat microglia cell lines were a generous gift from Dr. James R. Connor (Cheepsunthorn et al. 2001) and were maintained at 37°C in DMEM supplemented with 10% FBS, 50 U/mL penicillin and 50 µg/mL streptomycin in a humidified incubator with 5% CO2/95% air.
DA uptake assay
The DA uptake assay was performed on mesencephalic neuron-glia cultures 7–9 days after treatment, as we have previously described (Block et al. 2004). Briefly, cells were incubated in Krebs-ringer buffer (16 mM NaH2PO4, 16 mM NaH2PO4, 1.2 mM MgSO4, 1.3 mM EDTA, 4.7 nM KCL, 16 mM Na2HPO4) for 15 minutes at 37°C with 1 µM [3H] DA. Non-specific uptake was blocked for DA with 10 µM mazindol. After incubation, cells were washed three times with 1 mL/well of ice-cold Krebs-ringer buffer. Cells were then lysed with 0.5 mL/well of 1 N NaOH and mixed with 15 mL of scintillation fluid. Radioactivity was measured on a scintillation counter, where specific [3H] DA uptake was calculated by subtracting the mazindol counts from the wells without the uptake inhibitors.
Immunostaining
Microglia were stained with the polyclonal antibody raised against IBA-1 protein, as we have previously described (Levesque et al. 2011b).
TNFα and IL-1β ELISA
The production and release of Tumor Necrosis Factor α (TNFα) and Interleukin-1β (IL-1β) into the media was measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN), per manufacturer instructions.
Nitrite assay
As an indirect measure of nitric oxide production, the amount of nitrite accumulated in culture supernatant was determined with a colorimetric assay using Griess reagent [1% sulfanilamide, 2.5% H3PO4, 0.1% N- (1-naphthyl) ethylenediamine dihydrochloride] (Green et al. 1982) as previously reported (Block et al. 2004).
Hydrogen peroxide assay
Levels of hydrogen peroxide (H2O2) production in cell culture were determined as previously described (Werner 2003), with slight modifications (Levesque et al. 2011b).
Transmission electron microscopy
To observe DEP internalization, HAPI microglial cells were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, Fort Washington, PA) overnight at 4°C and then postfixed for 1 h at room temperature in 1% osmium tetroxide in 0.1 M cacodylate. After dehydration with graded ethanols and propylene oxide, cells were embedded in Polybed 812 (Polysciences, Warrington, PA). Thin sections were stained with 5% uranyl acetate followed by Reynold’s lead citrate and examined in a Tecnai 12 electron microscope (FEI Electron Optics, Eindhoven, The Netherlands) equipped with a digital Megaview III soft imaging system (SIS) and Windows 2000.
Statistical analysis
Data are expressed as the percentage of control where control values were set to 100%. The treatment groups are expressed as the mean ± SEM and statistical significance was assessed with a one or two-way Analysis of Variance followed by Bonferroni’s post hoc analysis with SPSS. A value of p< 0.05 was considered statistically significant.
RESULTS
Diesel exhaust particle components activate microglia
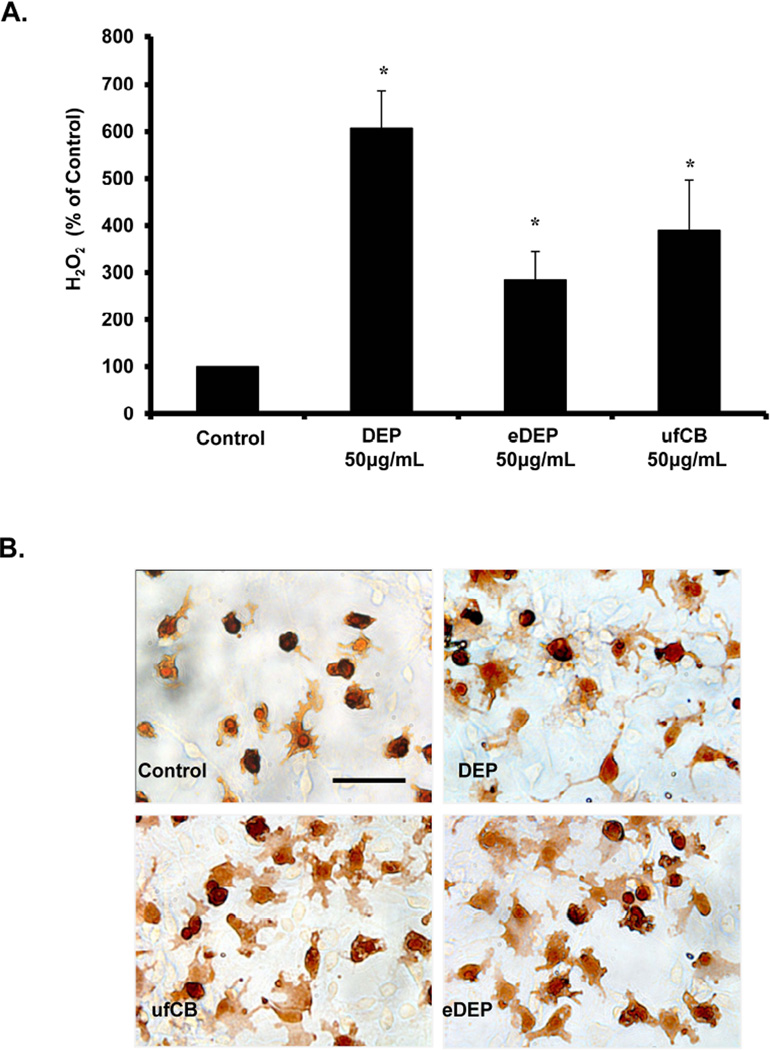
The nanometer-sized particulates and leachable components of DEP are proposed to translocate to the blood through the pulmonary capillary bed after inhalation to eventually reach the brain (Valavanidis et al. 2008). Until now, the components (carbon core or leachable chemicals) of DEP capable of activating microglia were unknown. Here, we show that DEP, ufCB (nanometer-sized carbon particles, similar to the carbon core of DEP), and eDEP (the extracts containing the adsorbed compounds on the DEP carbon core) all cause the production of H2O2 from microglia (p<0.05, Figure 1A) and induce an amoeboid, activated microglia morphology (Figure 1B) in vitro. Consistent with our previous studies demonstrating that DEP do not cause microglia to produce nitric oxide or cytokines in vitro (Levesque et al. 2011b, Block et al. 2004), we also found that DEP, ufCB, and eDEP failed to elevate TNFα, IL-1β, and nitrite in the culture media at any time point tested (3 and 24 hours post treatment) (p>0.05, data not shown). These data demonstrate that both the carbon core and the adsorbed chemicals of the DEP particle cause microglial activation.
Figure 1. Components of diesel exhaust particles (DEP) activate microglia.
(A) HAPI microglia cells were treated with DEP (50µg/ml), DEP extract (eDEP, from 50µg/ml DEP), and carbon black (ufCB, 50 µg/ml). The production of hydrogen peroxide (H2O2) was measured by the catalase-inhibitable fluorescence. Samples were run in triplicates and the data are the result of 4 independent experiments (n=4). Results are expressed as percent of control and represent the mean ± SEM. The raw data (fluorescence) for the control treatment range from 580 – 906 across experimental replicates. An asterisks indicates a significant difference from control (1 Way ANOVA, p<0.05). (B) Primary neuron-glia cultures were treated with DEP (50µg/ml), eDEP (from 50µg/ml DEP), and ufCB (50 µg/ml) for 9 hr and stained with the IBA-1 antibody. Microglial activation in response to the DEP components is depicted by an increase in number of stained cells, enlarged size of stained cells, and irregular amoeboid morphology. Representative images from the culture are shown from three independent experiments (n=3). Images were taken at 400× and the scale bar depicts 20µM.
Diesel exhaust particle components impair dopaminergic neuron function
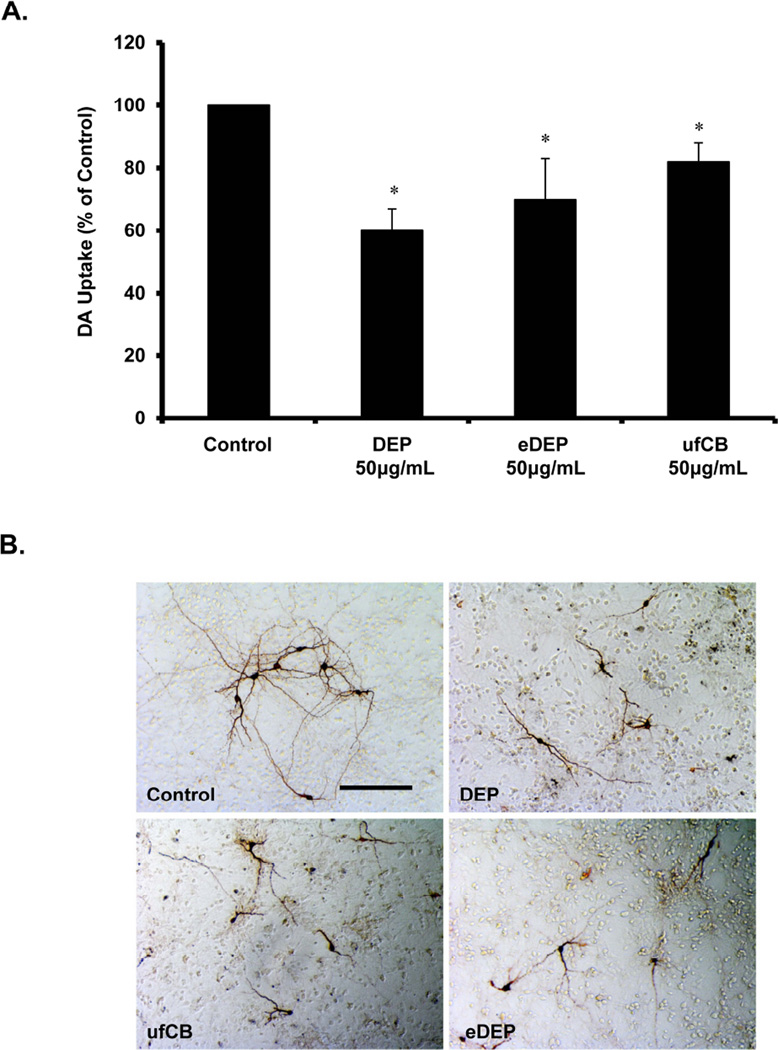
We have previously shown in vitro that DEP are selectively toxic to DA neurons through microglial NADPH oxidase activation and the consequent production of extracellular superoxide (Block et al. 2004). However, the mechanisms through which DEP cause the loss of DA neuron function are unknown. To begin to explore whether nanometer-sized particles or the chemical extracts were necessary for DEP-induced DA neuron damage/loss of function, we treated rat mesencephalic neuron-glia cultures with DEP, ufCB, and eDEP and assessed DA uptake 7 days later. Here, we show that both ufCB and eDEP impairs DA uptake in vitro (p<0.5, Figure 2), demonstrating that the microglial activation associated with each DEP component impairs DA neuron function.
Figure 2. Components of diesel exhaust particles (DEP) cause a loss of dopaminergic neuron function.
Primary mesencephalic neuron-glia cultures were treated with DEP (50µg/ml), DEP extract (eDEP, from 50µg/ml DEP), and carbon black (ufCB, 50 µg/ml). (A) Dopaminergic (DA) neuron function was measured in neuron-glia cultures 7–9 d after treatment with the [3H] DA uptake assay. Samples were run in triplicates and the data are the result of 5 independent experiments (n=5). Results are expressed as percent of control and are the mean ± SEM. The raw data (counts per second) for control values range from 8686– 5129 across experimental replicates. An asterisks indicates a significant difference from control (1 Way ANOVA, p<0.05). (B) Cultures were treated for 7–9 days and stained with the TH antibody. Dopaminergic neuron damage in response to the DEP components is depicted by shorter processes and fewer processes connecting the neurons. Representative images from the culture are shown from three independent experiments (n=3). Images were taken at 100× and the scale bar depicts 100µM.
Scavenger receptors mediate diesel exhaust particle internalization
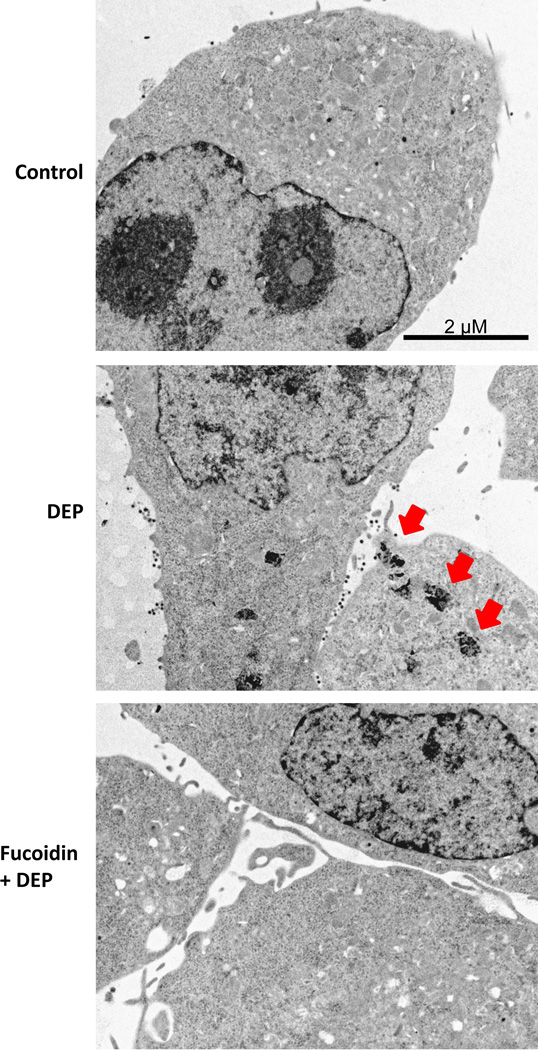
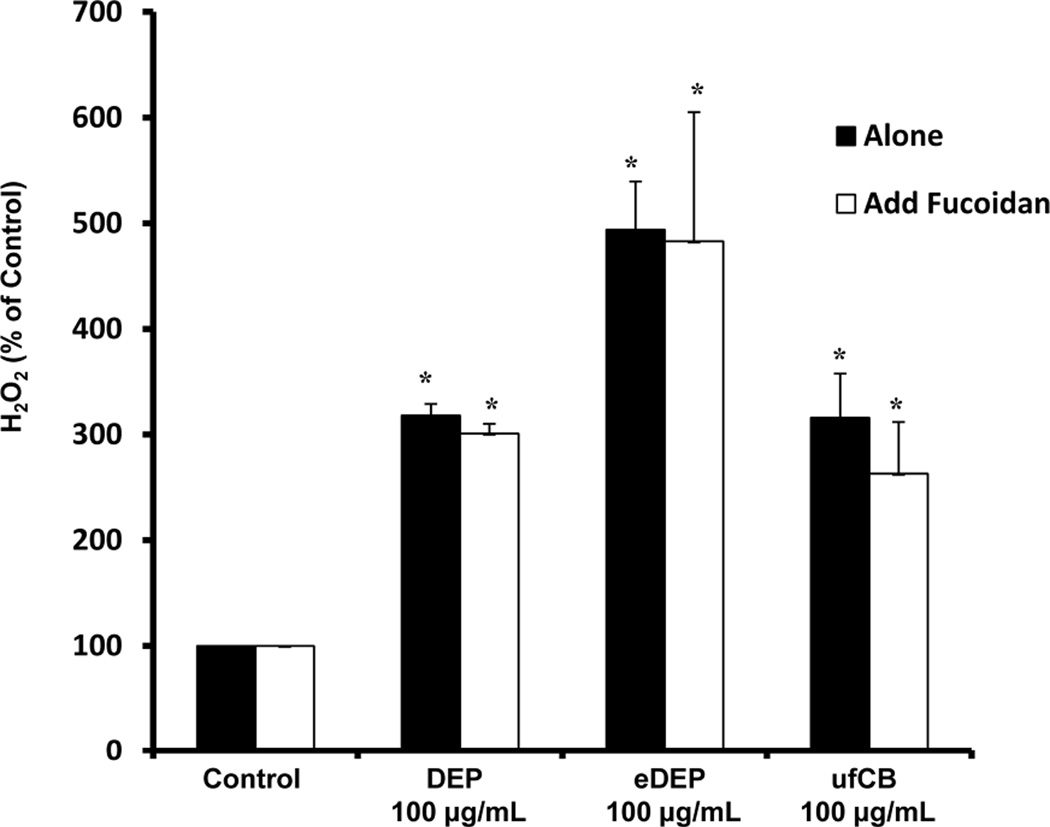
Scavenger receptors are essential for the innate immune response to pathogens and have been implicated in microglial activation, internalization, and ROS production in response to Aβ (Wilkinson & Khoury 2012). To determine the role of scavenger receptors in DEP-induced microglial activation, HAPI microglia were exposed to media alone (control), DEP (100 µg/ml), fucoidan (100 µg/ml), and fucoidan combined with DEP. The highest concentration of DEP was used to allow clear visualization of internalization. Figure 3 demonstrates that microglia internalize DEP, which is blocked by fucoidan, a competitive scavenger receptor antagonist (Patel et al. 2010). In addition, we show that DEP cause microglia to produce H2O2 (p<0.05), which fucoidan fails to inhibit (p>0.05, Figure 4). In fact, fucoidan failed to inhibit h2O2 production from eDEP and ufCB also (p>0.05, Figure 4). Thus, the microglial response to DEP is complex and the data support that the scavenger receptors responsible for DEP internalization are independent of the mechanism of H2O2 production.
Figure 3. Scavenger receptors mediate diesel exhaust particle (DEP) internalization.
Microglial cells were pretreated with the scavenger receptor inhibitor fucoidan (100µg/ml) for 30 min followed by DEP (100µg/ml) treatment. Electron micrographs representative of the HAPI microglia culture at 3 hr post-DEP treatment from 3 independent experiments are shown (n=3). Images were taken at 6000× to visualize internalization and the scale bar depicts 2µM. Red arrows indicate punctuate compartments in microglia containing particulate matter following DEP treatment. Fucoidan blocked DEP internalization, indicating a role for scavenger receptors in this process.
Figure 4. Scavenger receptors do not mediate diesel exhaust particle (DEP)-induced H2O2 production in microglia.
HAPI microglia cells were pretreated with the scavenger receptor inhibitor fucoidan (100µg/ml) for 30 min followed by DEP (100 µg/ml) DEP extract (eDEP, from 100 µg/ml DEP), and carbon black (ufCB, 100 µg/ml) treatment. The production of hydrogen peroxide (H2O2) was measured by the catalase-inhibitable fluorescence at 3 hr post-treatment. Fucoidan failed to affect DEP, eDEP, or ufCB -induced H2O2 production. Samples were run in triplicates and the data are the result of 3 independent experiments (n=3). Results are expressed as percent of control and are the mean ± SEM. The raw data (fluorescence) for the control treatment range from 601 –986 across experimental replicates. An asterisks indicates a significant difference from control (2 Way ANOVA, p<0.05; fucoidin treatment main effect, p<0.05; DEP treatment main effect, p<0.05).
MAC1 mediates diesel exhaust particle - induced H2O2 production and loss of DA neuron function
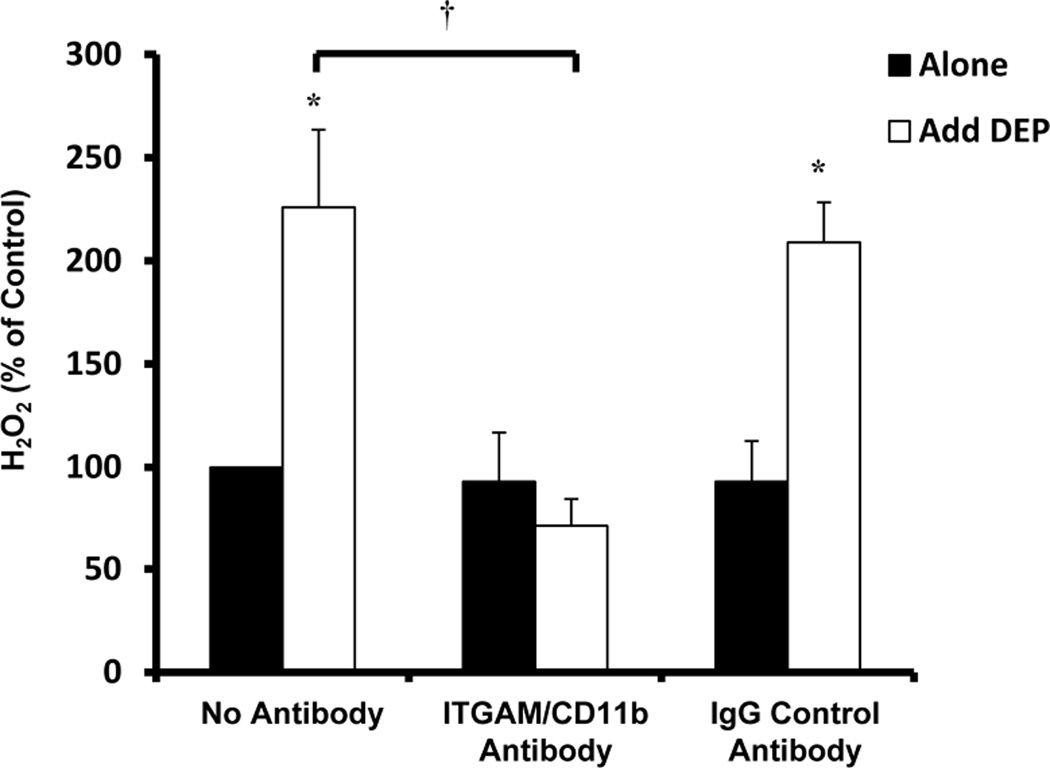
Macrophage-1 antigen (MAC1) has been implicated as a key pattern recognition receptor expressed selectively on cells of myeloid lineage, such as microglia (excluding neurons and astrocytes), that is important for how microglia are selectively toxic to DA neurons (Pei et al. 2007, Hu et al. 2008, Zhang et al. 2011). To begin to address the role of MAC1 in DEP-induced activation, we pre-treated HAPI microglia with media alone (no antibody), a blocking antibody against MAC1/CD11b (20 µg/ml), or a control mouse IgG antibody (20µg/ml) for 30 minutes and tested the ability of the antibodies to inhibit H2O2 production with the addition of DEP (50µg/ml) after 3 hours. Figure 5 depicts the effect of MAC1/CD11b blocking antibodies on DEP-induced H2O2 production in microglia cultures. More specifically, pre-treatment with the MAC1/CD11b antibody significantly reduced the production of H2O2 from HAPI microglia (p<0.05, Figure 5) and the mouse IgG control antibody had no effect (p>0.05, Figure 5).
Figure 5. MAC1 mediates diesel exhaust particle (DEP)-induced H2O2 production in microglia.
HAPI microglial cells were pretreated with the MAC1/CD11b inhibitor antibody (20 µg/ml) or mouse IgG control antibody (20 µg/ml) for 30 min followed by DEP (50 µg/ml) or LPS (200 ng/ml) treatment. The production of hydrogen peroxide (H2O2) was measured by the catalase-inhibitable fluorescence at 3 hr post-treatment. The MAC1/CD11b antibody inhibited DEP-induced H2O2 production. Samples were run in triplicates and the data are the result of three independent experiments (n=3). Results are expressed as percent of control and are the mean ± SEM. The raw data (fluorescence) for the control treatment range from 580 – 906 across experimental replicates. An asterisks indicates a significant difference from control (p<0.05) and a “†” indicates a significant difference between the mouse strains (2 Way ANOVA, p<0.05; strain by treatment interaction, p<0.05).
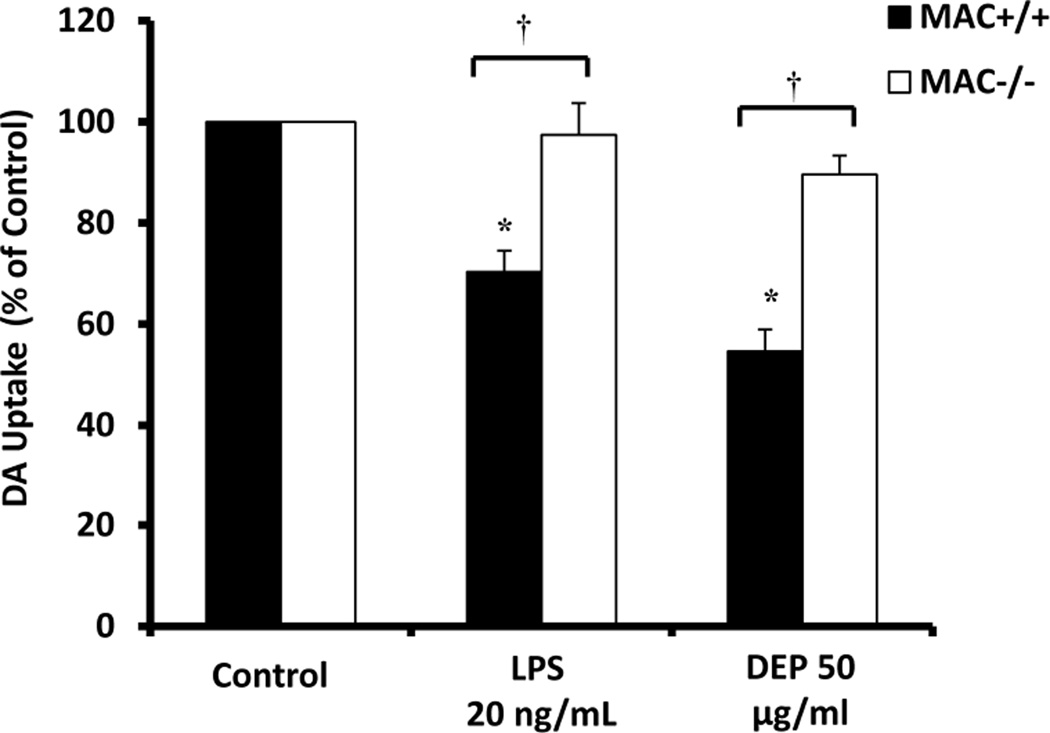
We next sought to discern the role of MAC1 in DEP-induced loss of DA neuron function. Mesencephalic neuron-glia cultures from MAC1+/+ and MAC1−/− mice were treated with media alone (control) lipopolysaccharide (LPS, 20ng/ml) as a positive control for microglia-mediated neurotoxicity, or DEP (50µg/ml). DA uptake was performed 7 –9 days later. Figure 6 demonstrates that while DEP and LPS decreased DA uptake in MAC1+/+ cultures, there was no significant effect in MAC1−/− cultures (p<0/05). Together, these data support that MAC1 is critical for DEP-induced H2O2 in microglia and loss of DA neuron-function.
Figure 6. MAC1 mediates diesel exhaust particle (DEP)-induced loss of dopaminergic neuron function.
The effect of diesel exhaust particles on loss of DA neuron function was compared in mesencephalic neuron-glia cultures from MAC1−/− and MAC1+/+ mice. Lipopolysaccharide (LPS) was used as a positive control for microglia-induced loss of neuronal function and MAC1-specific DA neuron damage. Loss of DA neuron function was measured at 7 days post treatment using the [3H] DA uptake assay. Results are expressed as percent of control and are the mean ± SEM. Samples were run in triplicates and the data are the result of 3 independent experiments (n=3). The raw data (counts per second) for control values range from 1429– 4811 across experimental replicates. An asterisks indicates a significant difference from control (p<0.05) and a “†” indicates a significant difference between the mouse strains (2 Way ANOVA, p<0.05; strain × treatment interaction, p<0.05).
DISCUSSION
Accumulating evidence points to microglial activation as a contributing factor to neuropathology, neuroinflammation, and oxidative stress in response to air pollution exposure, but the specific responses and the mechanisms driving how microglia become activated are as of yet unresolved. Recent studies have identified PM from urban air pollution in human brains (Calderon-Garciduenas et al. 2008b) and animal studies have revealed that various inhaled nanoparticles translocate to the brain (Lucchini et al. 2011), supporting that inhaled PM interacts with cells in the brain parenchyma. Here, we addressed how the particle components of DE and the chemical compounds contained on the particle itself activate microglia to impact DA neuron function in vitro. We also began to explore the identity of the pattern recognition receptors necessary for microglia to respond to DEP and the consequence for DA neuron function.
To begin to understand how DEP activates microglia, we assessed the role of the chemical extracts from DEP (eDEP) and the nanometer-sized particle (ufCB) on microglial activation and DA function, when compared to DEP. Figure 1 shows that both eDEP and ufCB elevated H2O2 production and induced an amoeboid, activated microglia morphology, similar to DEP. Figure 2 demonstrates that similar to DEP, both eDEP and ufCB reduced DA uptake and neuron morphology indicative of damage, indicating that both components impair DA neuron function in vitro. Together, these data indicate that DEP are a complex trigger of microglial activation, where multiple characteristics of DEP have the potential to activate microglia and impair DA neuron function. Notably, there are over 300 chemical compounds adsorbed on DEP (Ma & Ma 2002), many of which have the potential to be neurotoxic. As such, there is a significant need for future research to further refine mechanistic studies by identifying and quantitating the amount of PM and associated chemicals that reach the brain in vivo upon DE exposure.
Microglia actively survey the brain environment (Nimmerjahn et al. 2005) and rapidly respond to large molecular patterns (e.g. α synuclein, LPS, neuromelanin, and Aβ) that trigger a pro-inflammatory response with pattern recognition receptors (Block et al. 2007). Scavenger receptors are pattern recognition receptors expressed on multiple cell types, including microglia, and are broadly defined as a family of molecules that share the ability to bind polyanionic ligands, which include both pathogens/particles and ligands of self-origin (Wilkinson & Khoury 2012). Class A scavenger receptors are a subgroup that are essential for host defense against several bacterial and viral pathogens (Wilkinson & Khoury 2012). For example, class A scavenger receptors have been implicated in microglial activation, internalization, and ROS production in response to Aβ (Wilkinson & Khoury 2012), emphasizing the potential role of this receptor subtype in neurotoxic microglial activation. Here, we demonstrate that while scavenger receptors regulate microglial internalization of DEP (Figure 3), these receptors have no effect on DEP-induced H2O2 production (Figure 4). The importance of scavenger receptors for DEP clearance without reactive oxygen species production supports a beneficial role for these receptors, similar to what has been found for the microglial response to both LPS (Pei et al. 2007) and α synuclein (Zhang et al. 2007).
The MAC1 pattern receptor is selectively expressed on cells of myeloid lineage, such as microglia (Akiyama & McGeer 1990), binds LPS (Wright & Jong 1986, Wright et al. 1989), and was previously identified as a TLR4-independent receptor for LPS in phagocytes (Perera et al. 1997), including microglia (Pei et al. 2007). We have previously shown that MAC1 regulates neurotoxic reactive microglia microgliosis in response to the DA neurotoxin MPTP (Hu et al. 2008), is responsible for LPS-induced extracellular super-oxide production in microglia (Pei et al. 2007), and is a component of LPS-induced DA neurotoxicity (Pei et al. 2007). In addition, other labs have also shown that microglial MAC1 plays a role in α synuclein-induced (Zhang et al. 2007) and neuromelanin-induced (Zhang et al. 2011) DA neurotoxicity, further supporting a key role for MAC1 in microglia-mediated neurotoxicity. In the current study, we demonstrate that MAC1 is essential for DEP-induced H2O2 production (Figure 5) and loss of DA function (Figure 6), emphasizing a key role for both microglia and this receptor in the deleterious effects of DEP.
In summary, DEP are a complex trigger of microglial activation that cause activated microglial morphology and reactive oxygen species production (superoxide and H2O2), without cytokine (TNFα and IL-1β) or nitric oxide production. Data support that both the adsorbed compounds (eDEP) and the carbon particles (ufCB) are capable of activating microglia and may contribute to the microglial response to DEP. Further, scavenger receptors were shown to mediate internalization and clearance of DEP without H2O2 production, which is likely beneficial. However, MAC1 was shown to mediate the microglia H2O2 response to DEP and the associated loss of DA neuron function, demonstrating a deleterious role for this pattern recognition receptor. While other pattern recognition receptors may also contribute, these data support that DEP can exert deleterious effects through microglia and that the MAC1 pattern recognition receptors may be key to this process, providing much needed insight into the mechanisms through which air pollution can impact the brain.
ACKNOWLEDGEMENTS
This research was supported by the National Institute of Environmental Health Sciences/the National Institute of health [Grant number1R01ES016951]. The authors thank Thurn Sams and Maria Bright for their assistance with maintenance and breeding of timed pregnant animals.
Defining abbreviations
- PM
particulate matter
- DE
diesel exhaust
- DEP
diesel exhaust particles
- eDEP
diesel exhaust particles extract
- ufCB
ultrafine carbon black
- CNS
central nervous system
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- IL-1β
interleukin-1β
- DA
dopaminergic
- MAC1
macrophage-1 antigen
- H2O2
hydrogen peroxide
- IBA-1
ionized calcium binding adaptor molecule
- CD11b
cluster of differentiation molecule 11b
- HAPI
highly aggressive proliferating immortalized
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- Bos I, De Boever P, Emmerechts J, et al. Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal Toxicol. 2012;24:676–686. doi: 10.3109/08958378.2012.714004. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Franco-Lira M, Henriquez-Roldan C, et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol. 2010;62:91–102. doi: 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Kavanaugh M, Block M, et al. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis. 2012;28:93–107. doi: 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008a;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008b;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Campbell A, Boere AJ, McLean SG, Duffin R, Krystek P, Gosens I, Miller MR. The biological effects of subacute inhalation of diesel exhaust following addition of cerium oxide nanoparticles in atherosclerosis-prone mice. Environ Res. 2012;115:1–10. doi: 10.1016/j.envres.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Chen JC, Schwartz J. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology. 2009;30:231–239. doi: 10.1016/j.neuro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Cordier S, Monfort C, Filippini G, et al. Parental exposure to polycyclic aromatic hydrocarbons and the risk of childhood brain tumors: The SEARCH International Childhood Brain Tumor Study. Am J Epidemiol. 2004;159:1109–1116. doi: 10.1093/aje/kwh154. [DOI] [PubMed] [Google Scholar]
- Cruts B, van Etten L, Tornqvist H, Blomberg A, Sandstrom T, Mills NL, Borm PJ. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part Fibre Toxicol. 2008;5:4. doi: 10.1186/1743-8977-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- Donnan GA, McNeil JJ, Adena MA, Doyle AE, O'Malley HM, Neill GC. Smoking as a risk factor for cerebral ischaemia. Lancet. 1989;2:643–647. doi: 10.1016/s0140-6736(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P, Tajuba J, Lippmann M, Chen LC, Veronesi B. Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Guxens M, Aguilera I, Ballester F, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120:144–149. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M, Sunyer J. A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly. 2012;141:w13322. doi: 10.57187/smw.2012.13322. [DOI] [PubMed] [Google Scholar]
- Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64:439–445. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Long CM, Lapin CA, Hamade AK, Valberg PA. Diesel exhaust particulate (DEP) and nanoparticle exposures: what do DEP human clinical studies tell us about potential human health hazards of nanoparticles? Inhal Toxicol. 2010;22:679–694. doi: 10.3109/08958371003758823. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Immunol. 2008;181:7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21(Suppl 1):55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011a;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011b;119:1149–1155. doi: 10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wang K, Gao HM, Mandavilli B, Wang JY, Hong JS. Molecular consequences of activated microglia in the brain: overactivation induces apoptosis. J Neurochem. 2001;77:182–189. doi: 10.1046/j.1471-4159.2001.t01-1-00216.x. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Dorman DC, Elder A, Veronesi B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology. 2012;33:838–841. doi: 10.1016/j.neuro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Ma JK. The dual effect of the particulate and organic components of diesel exhaust particles on the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2002;20:117–147. doi: 10.1081/GNC-120016202. [DOI] [PubMed] [Google Scholar]
- Morales E, Julvez J, Torrent M, de Cid R, Guxens M, Bustamante M, Kunzli N, Sunyer J. Association of early-life exposure to household gas appliances and indoor nitrogen dioxide with cognition and attention behavior in preschoolers. Am J Epidemiol. 2009;169:1327–1336. doi: 10.1093/aje/kwp067. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Davis DA, Iwata N, et al. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect. 2011;119:1003–1009. doi: 10.1289/ehp.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Ovrevik J, Lag M, Holme JA, Schwarze PE, Refsnes M. Cytokine and chemokine expression patterns in lung epithelial cells exposed to components characteristic of particulate air pollution. Toxicology. 2009;259:46–53. doi: 10.1016/j.tox.2009.01.028. [DOI] [PubMed] [Google Scholar]
- Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug Chem. 2010;21:2250–2256. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Pang H, Qian L, et al. MAC1 mediates LPS-induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55:1362–1373. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- Power MC, Weisskopf MG, Alexeeff SE, Coull BA, Spiro A, 3rd, Schwartz J. Traffic-related air pollution and cognitive function in a cohort of older men. Environ Health Perspect. 2011;119:682–687. doi: 10.1289/ehp.1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: a literature review. J Expo Sci Environ Epidemiol. 2009;19:443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft U, Schikowski T, Sugiri D, Krutmann J, Kramer U. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. Environ Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Schwab C, McGeer PL. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- Stoeger T, Reinhard C, Takenaka S, Schroeppel A, Karg E, Ritter B, Heyder J, Schulz H. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ Health Perspect. 2006;114:328–333. doi: 10.1289/ehp.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. Am J Epidemiol. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, Takeda K. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol. 2010;7:7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Airborne inflammatory factors: "from the nose to the brain". Front Biosci (Schol Ed) 2010;2:135–152. doi: 10.2741/s52. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- van Berlo D, Albrecht C, Knaapen AM, et al. Comparative evaluation of the effects of short-term inhalation exposure to diesel engine exhaust on rat lung and brain. Arch Toxicol. 2010;84:553–562. doi: 10.1007/s00204-010-0551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21:689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Martinez D, Aguilera I, et al. Indoor air pollution from gas cooking and infant neurodevelopment. Epidemiology. 2012;23:23–32. doi: 10.1097/EDE.0b013e31823a4023. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang J, Zeng X, Zeng Y, Chen S. Association of traffic-related air pollution with children's neurobehavioral functions in Quanzhou, China. Environ Health Perspect. 2009;117:1612–1618. doi: 10.1289/ehp.0800023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. Determination of cellular H2O2 production. Sci STKE. 2003;2003:PL3. doi: 10.1126/stke.2003.168.pl3. [DOI] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172:219–227. doi: 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K, Khoury E. Microglial Scavenger Receptors and Their Roles in the Pathogenesis of Alzheimer’s Disease. International Journal of Alzheimer's Disease. 2012 doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Yamamoto S, Fujitani Y, Hirano S, Fujimaki H. Nanoparticle-rich diesel exhaust affects hippocampal-dependent spatial learning and NMDA receptor subunit expression in female mice. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.590904. [DOI] [PubMed] [Google Scholar]
- Wright SD, Jong MT. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Mizuo K, Moriya N, Oshio S, Sugawara I, Takeda K. Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neurosci Lett. 2009;449:38–41. doi: 10.1016/j.neulet.2008.09.085. [DOI] [PubMed] [Google Scholar]
- Zanchi AC, Saiki M, Saldiva PH, Barros HM, Rhoden CR. Hippocampus lipid peroxidation induced by residual oil fly ash intranasal instillation versus habituation to the open field. Inhal Toxicol. 2010;22:84–88. doi: 10.3109/08958370902936931. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dallas S, Zhang D, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- Zhang W, Phillips K, Wielgus AR, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease. Neurotox Res. 2011;19:63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]