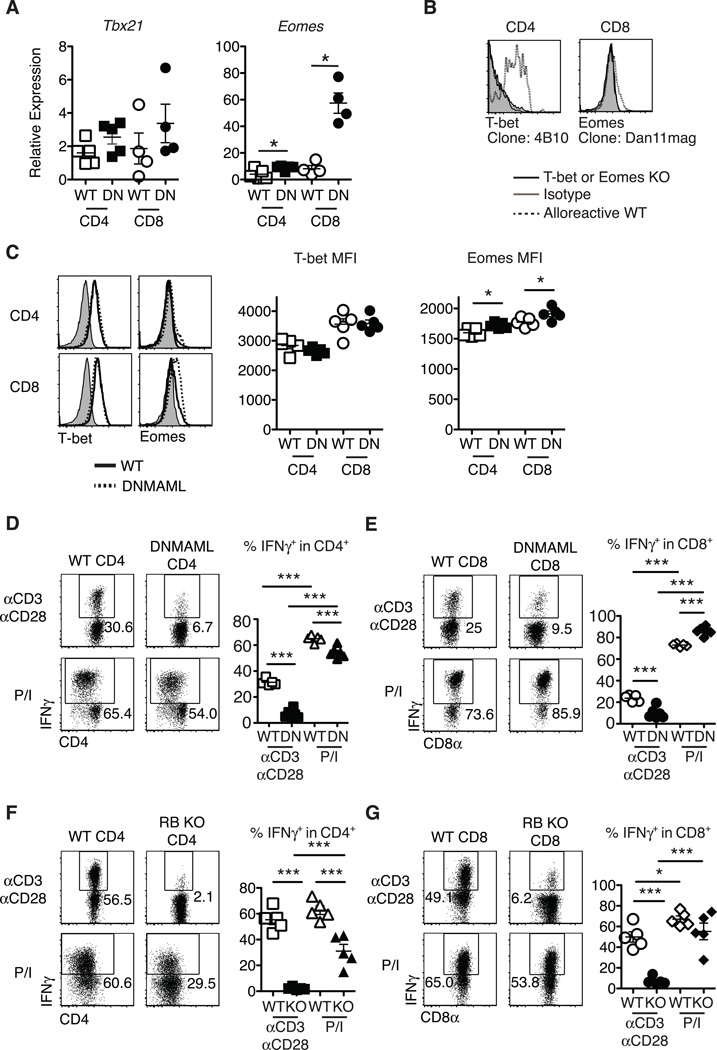
Figure 3.
Preserved T-bet and enhanced Eomesodermin expression in alloreactive Notch-deprived CD4+ and CD8+ T cells, allowing restoration of IFNγ production after treatment with PMA and ionomycin. Lethally irradiated BALB/c mice (900 rads) were transplanted with B6-CD45.1 TCD BM (5×106 cells) and splenocytes (10×106 cells) from WT or DNMAML B6 mice. (A) Preserved Tbx21 mRNA (encoding T-bet) and enhanced Eomes transcripts in DNMAML CD4+ and CD8+ T cells. Donor-derived H-2Kb+H-2Kd–CD45.2+ CD4+ and CD8+ T cells were sort-purified and subjected to qRT-PCR (day 14, n=4–5 mice, representative of 3 experiments); (B) Specific detection of T-bet and Eomesodermin in alloreactive T cells with anti-T-bet (4B10) and anti-Eomesodermin (Dan11mag) antibodies. Splenocytes from WT, B6.129S6-Tbx21tm1Glm/J or Eomesf/fxCd4-Cre mice were transplanted into irradiated BALB/c recipients. Histograms show intracellular staining with isotype control or specific antibodies in donor-derived H-2Kb+H-2Kd–CD45.2+ CD4+ or CD8+ T cells (day 5) (n=2); (C) Representative intracellular flow cytometry plots and mean fluorescence intensity (MFI) for T-bet and Eomesodermin expression in alloreactive WT and DNMAML CD4+ and CD8+ T cells (day 14, n=4–5 mice, representative of 4 experiments); (D-G) At day 5 after transplantation, spleen and lymph node cells were incubated for 6 hours with either anti-CD3/anti-CD28 (2.5 µg/ml each), or PMA and ionomycin (50 ng/ml and 500 ng/ml, respectively). Percent IFNγ+ cells as measured by intracellular flow cytometry in WT and DNMAML donor-derived (D) CD4+ and (E) CD8+ T cells, or WT and CSL/RBP-Jk-deficient donor-derived (F) CD4+ and (G) CD8+ T cells (n=5 mice/group, representative of >2 experiments). Representative flow cytometry plots are shown. Numbers indicate the percentage of cells in each quadrant. * p<0.05; *** p<0.001.