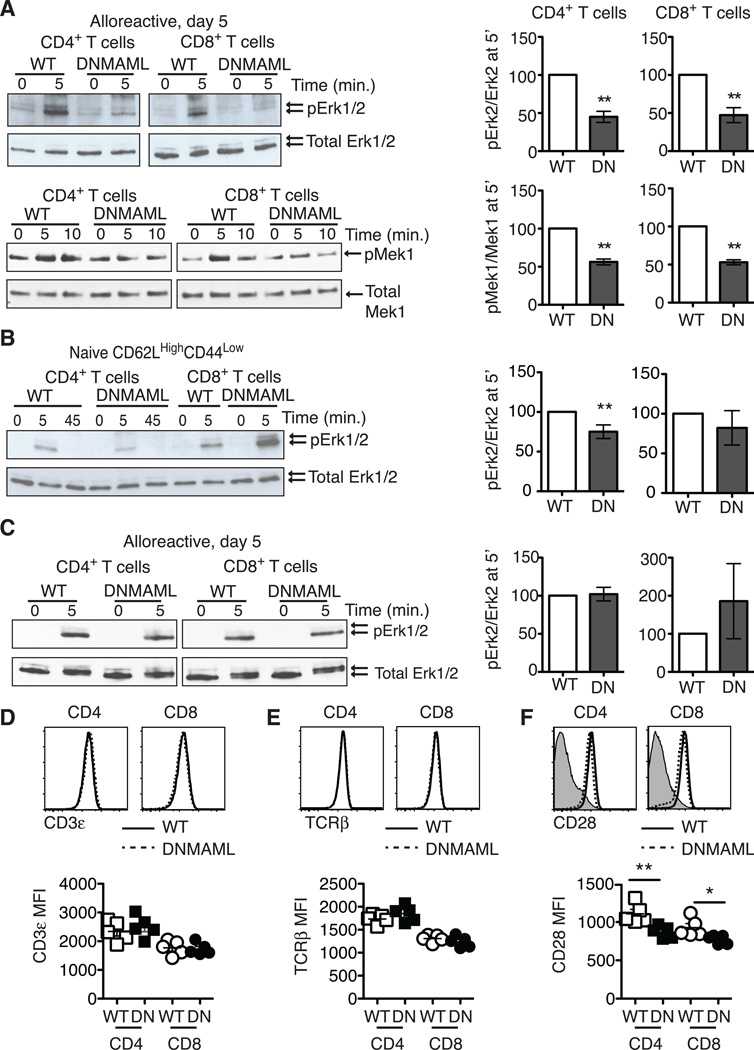
Figure 4.
Alloreactive Notch-deprived CD4+ and CD8+ T cells have an acquired defect in Ras/MAPK pathway activation that is rescued by PMA. (A) WT or DNMAML B6 splenocytes were transplanted into lethally irradiated BALB/c recipients. On day 5, H-2Kb+H-2Kd– alloreactive WT and DNMAML CD4+ and CD8+ T cells were sort-purified and restimulated in vitro for 5–10’ at 37°C with anti-CD3/anti-CD28 and IgG crosslinking. Baseline activation was assessed by incubating cells at 37°C with IgG crosslinker alone (0’ time point). Phosphorylated Erk1/2 and Mek1 were detected by Western blotting as compared to total Erk1/2 and Mek1; (B) Naïve CD62LHighCD44Low CD4+ and CD8+ T cells were sort-purified from WT and DNMAML mice. Mek1 phosphorylation was assessed after anti-CD3/CD28 restimulation; (C) Sort-purified, day 5 alloreactive WT and DNMAML CD4+ or CD8+ T cells were restimulated ex vivo with PMA for 5’ (or DMSO as negative control). In all experiments, the abundance of phosphorylated proteins was measured by densitometry relative to total protein levels. WT T cells were set to 100% (n=2–4 individual experiments, 6 mice/group in each experiment); (D) cell surface CD3, (E) TCRβ, and (F) CD28 levels were assessed in alloreactive WT and DNMAML CD4+ and CD8+ T cells on day 5 post-transplantation. Representative flow cytometry plots and mean fluorescence intensity are shown. **p<0.01.