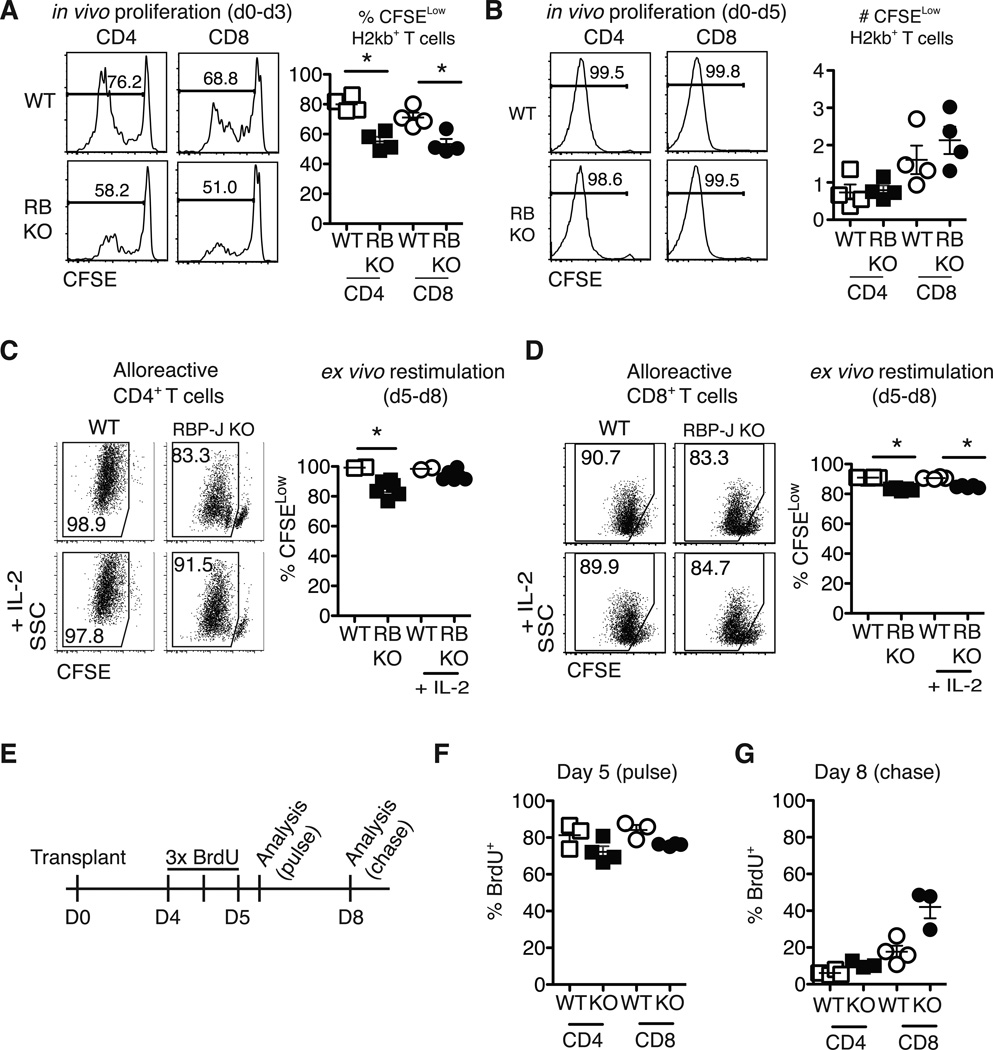
Figure 6.
Notch-deprived alloreactive CD4+ and CD8+ T cells have a preserved initial proliferative burst but subsequent reduced proliferation in vitro and in vivo. CFSE-labeled T cells from WT or Rbpjf/f x Cd4-Cre mice (RB KO, lacking all CSL/RBP-Jk-mediated Notch signals) were transplanted into lethally irradiated BALB/c recipients (900 rads). Flow cytometry plots show CFSE dilution at (A) day 3 and (B) day 5 in donor-derived H-2Kb+H-2Kd– CD4+ and CD8+ T cells. WT or RBP-Jk KO T cells were transplanted into lethally irradiated BALB/c recipients. On day 5, purified WT and RBP-Jk KO (C) CD4+ or (D) CD8+ T cells were CFSE-labeled and restimulated in vitro for 3 days with anti-CD3/CD28 +/− IL-2. Division history of donor-derived H-2Kb+H-2Kd– B6 T cells was measured by flow cytometry (n=6–7 mice/group in each experiment, representative of >2 experiments); (E) Design of BrdU pulse-chase experiment. Lethally irradiated BALB/c recipients were transplanted with splenocytes from WT or RBP-Jk KO mice. Between days 4 and 5, transplant recipients received 3 doses of BrdU 12 hours apart; (F) Four hours after the last BrdU injection, mice were euthanized for day 5 BrdU incorporation analysis (pulse); (G) At day 8 after transplantation, residual BrdU content was assessed (chase). n=5 mice/group in each experiment, representative of 2 experiments. *p<0.05