Abstract
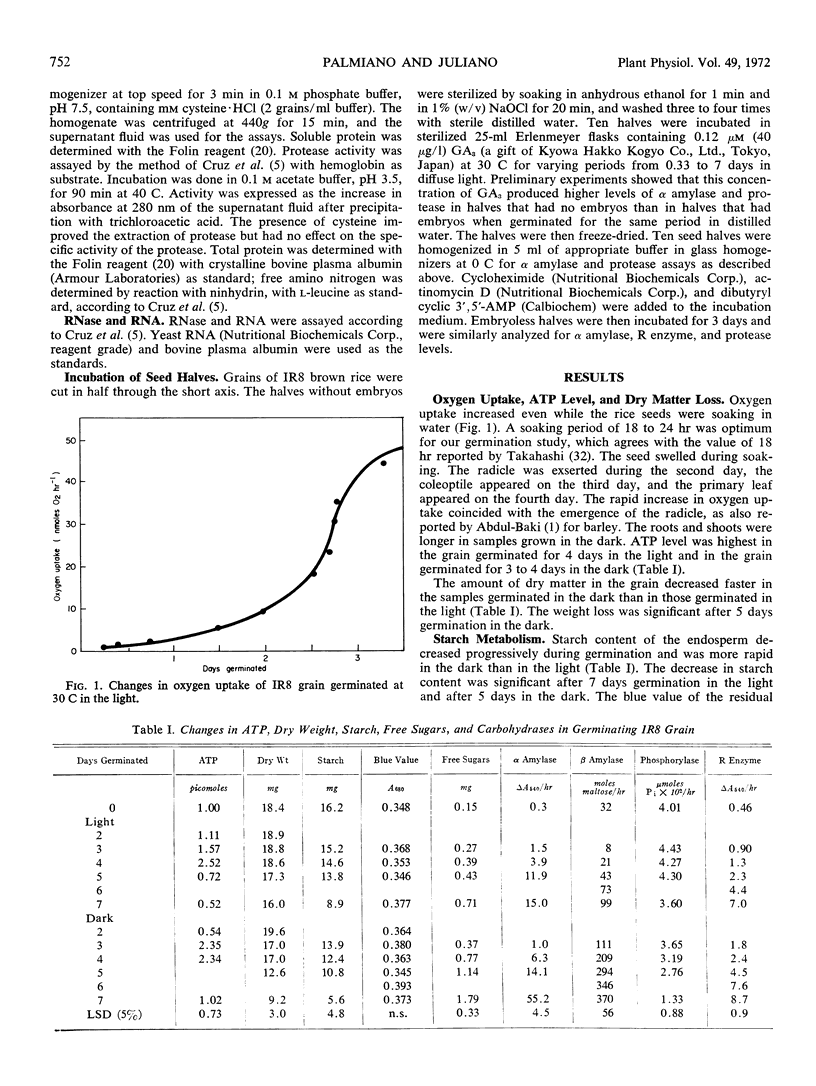
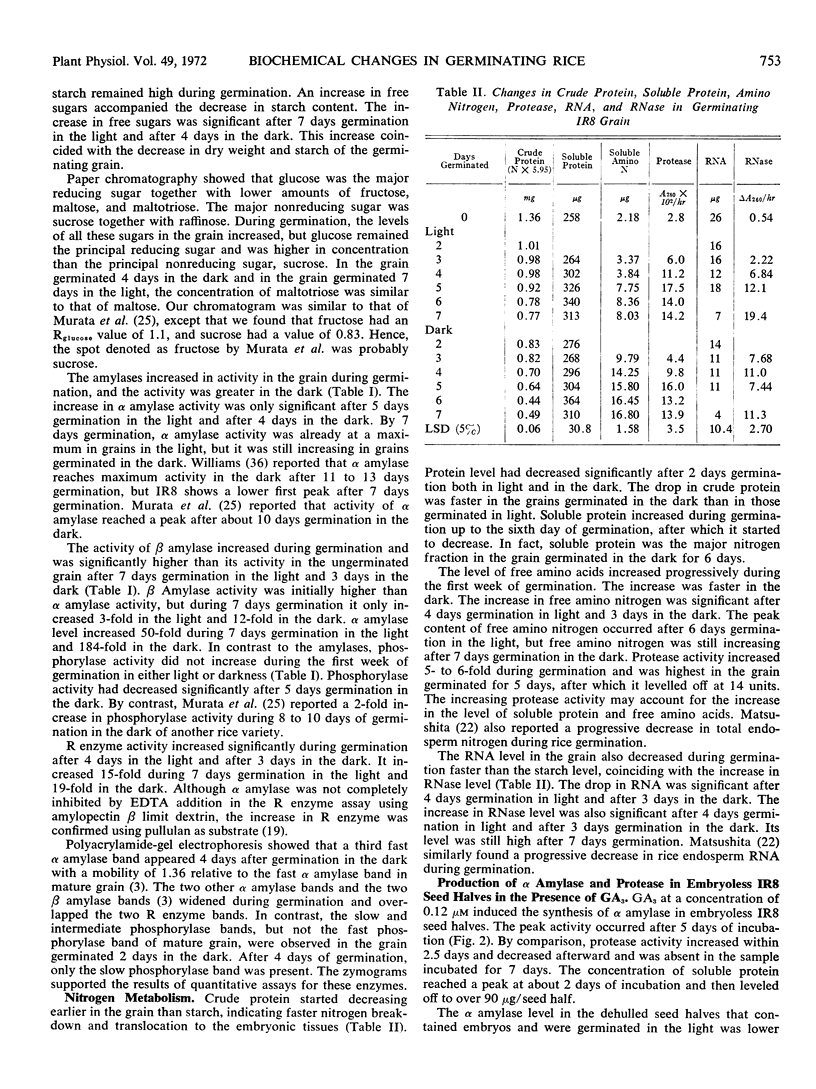
Changes in the content of starch, protein, and RNA and in the activity of their hydrolases in the rice endosperm (Oryza sativa L., variety IR8) were determined during the first week of germination without added nutrient both in the dark and in the light. Changes were generally more rapid in the dark than in the light. Oxygen uptake and RNase activity started to increase and the root protruded on the second day, followed by the coleoptile on the third day, and the primary leaf on the fourth day. ATP level was at a maximum on the fourth day. The activity of amylases and R enzyme increased progressively, but that of phosphorylase tended to decrease during starch degradation. A new α amylase isozyme band appeared during germination. Glucose was the major product of starch degradation. Sucrose, maltose, maltotriose, raffinose, and fructose were also detected. Protease activity reached a maximum on the fifth or sixth day and closely paralleled the increase in soluble amino N and soluble protein.
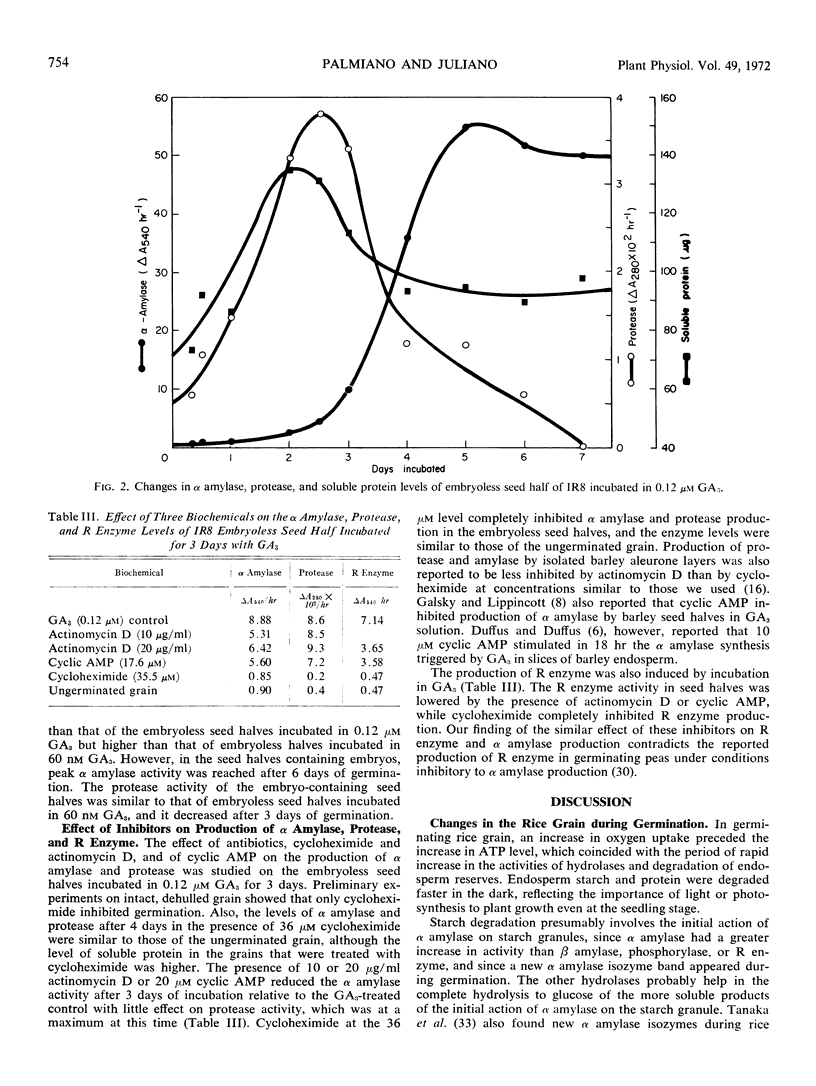
In embryoless seed halves with 0.12 μM gibberellin As, peak protease activity occurred in 2.5 days and peak α amylase activity on the fifth day of incubation. The production of α amylase, protease, and R enzyme was inhibited by 40 μM cycloheximide, but only α amylase and R enzyme were inhibited by 20 μg/ml actinomycin D.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A. Metabolism of barley seed during early hours of germination. Plant Physiol. 1969 May;44(5):733–738. doi: 10.1104/pp.44.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addanki S., Sotos J. F., Rearick P. D. Rapid determination of picomole quantities of ATP with a liquid scintillation counter. Anal Biochem. 1966 Feb;14(2):261–264. doi: 10.1016/0003-2697(66)90135-7. [DOI] [PubMed] [Google Scholar]
- Baun L. C., Palmiano E. P., Perez C. M., Juliano B. O. Enzymes of starch metabolism in the developing rice grain. Plant Physiol. 1970 Sep;46(3):429–434. doi: 10.1104/pp.46.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L. J., Cagampang G. B., Juliano B. O. Biochemical factors affecting protein accumulation in the rice grain. Plant Physiol. 1970 Nov;46(5):743–747. doi: 10.1104/pp.46.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffus C. M., Duffus J. H. A possible role for cyclic AMP in gibberellic acid triggered release of alpha-amylase in barley endosperm slices. Experientia. 1969 Jun 15;25(6):581–581. doi: 10.1007/BF01896521. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Dure L., 3rd Synthesis of a protease in germinating cotton cotyledons catalzed by mRNA synthesized during embryogenesis. Biochem Biophys Res Commun. 1969 Aug 22;36(5):705–710. doi: 10.1016/0006-291x(69)90667-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Scandalios J. G., Varner J. E. Multiple forms of amylase induced by gibberellic acid in isolated barley aleurone layers. Plant Physiol. 1970 Apr;45(4):367–371. doi: 10.1104/pp.45.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Varner J. E. Gibberellic Acid-induced synthesis of protease by isolated aleurone layers of barley. Plant Physiol. 1967 Nov;42(11):1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano B. O., Varner J. E. Enzymic degradiation of starch granules in the cotyledons of germinating peas. Plant Physiol. 1969 Jun;44(6):886–892. doi: 10.1104/pp.44.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski E., Bushuk W. Detection of multiple forms of proteolytic enzymes by starch gel electrophoresis. Can J Biochem. 1968 Oct;46(10):1317–1320. doi: 10.1139/o68-197. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee E. Y., Marshall J. J., Whelan W. J. The substrate specificity of amylopectin-debranching enzymes from sweet corn. Arch Biochem Biophys. 1971 Apr;143(2):365–374. doi: 10.1016/0003-9861(71)90223-2. [DOI] [PubMed] [Google Scholar]
- Murata T. Enzymic mechanism of starch breakdown in germinating rice seeds I. An analytical study. Plant Physiol. 1968 Dec;43(12):1899–1905. doi: 10.1104/pp.43.12.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Kono Y., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds II. Scutellum as the Site of Sucrose Synthesis. Plant Physiol. 1969 May;44(5):765–769. doi: 10.1104/pp.44.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Henningsen K. W. Enzymes associated with protein bodies isolated from ungerminated barley seeds. Plant Physiol. 1969 Nov;44(11):1488–1498. doi: 10.1104/pp.44.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Ito T., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: III. alpha-Amylase Isozymes. Plant Physiol. 1970 Nov;46(5):650–654. doi: 10.1104/pp.46.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]


