Figure 6.
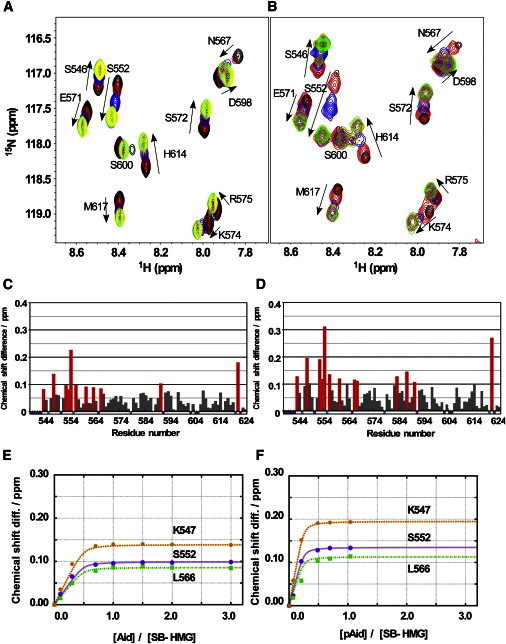
NMR titration experiments using the 15N-labeled SB-HMG fragment. (A) Chemical-shift changes in the titration of nonphosphorylated AID fragment (AID) to 15N-labeled SB-HMG; AID/SB-HMG molar ratios were 0.0 (black), 0.3 (red), 0.7 (blue), 1.0 (orange), 1.5 (pink), 2.0 (green), and 3.0 (yellow). (B) The corresponding spectral changes observed for phosphorylated AID (pAID); pAID/SB-HMG ratios were 0.00 (black), 0.07 (red), 0.21 (blue), 0.49 (orange), 0.70 (pink), and 1.04 (green). (C and D) Histograms of the chemical-shift differences in SB-HMG upon binding with AID (C) and pAID (D). Chemical-shift differences are plotted against residue numbers of SB-HMG. Red bars indicate that the chemical-shift differences are over the average plus one standard deviation. Short bars in cyan and purple along the x axis represent prolines and the residues for which assignment information was missing. Chemical-shift changes are shown for the representative residues in SB-HMG in the titrations with AID (E) and pAID (F). The numerically determined dissociation constant, KD, and number of binding sites, n, for the AID and pAID titration experiments were KD = 9.7 ± 0.3 μM, n = 2.0 ± 0.1 and KD = 1.01 ± 0.02 μM, n = 3.9 ± 0.1, respectively. Values for KD and n were determined by global fitting using the residues marked by red bars in C and D (see Supporting Material).
