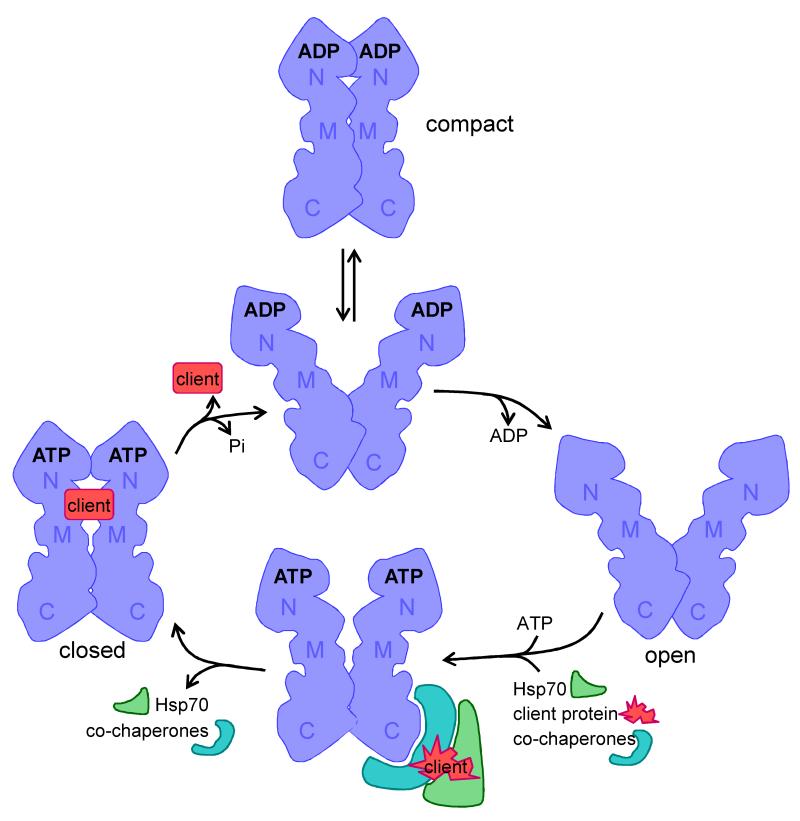
Figure 1. The Hsp90 chaperone cycle.
The shape of the Hsp90 monomer is adapted from Pearl et al. (2008)26, illustrating the amino- (N), middle (M) and carboxy-terminal (C) domains. The Hsp90 chaperone cycle has been reviewed recently17, 23. Hsp90 acts as a dimer that can take up various dynamic conformations in its ADP bound-state in which the amino terminal domains can be apart or closely associated (compact form). The Hsp90 dimer takes up an open state following release of ADP. The rapid association with ATP is associated with interactions with co-chaperones, Hsp70 and client proteins. Different co-chaperones can associate with different domains of the Hsp90 molecule and mediate interactions with distinct client proteins17,23. This is followed by the slow formation of a closed complex and the release of Hsp70 and co-chaperones. Then the folded client protein is released and the ATP is hydrolysed.