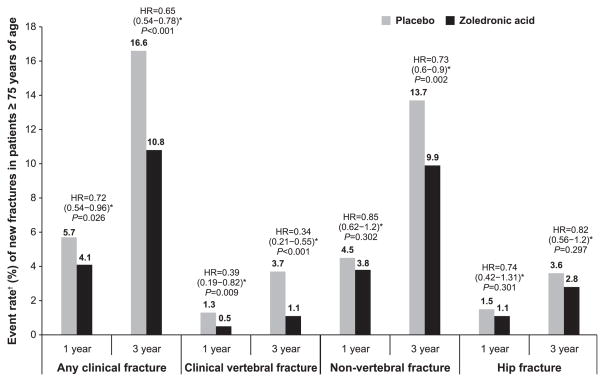
Figure 1.
Event rate of new fractures in patients receiving zoledronic acid (ZOL) 5 mg once yearly and those receiving placebo at 1 and 3 years. *Hazard ratio (HR) (95% confidence interval) of ZOL versus placebo computed from the Cox proportional hazards regression model stratified according to study with treatment as a factor within the subgroup. †Event rate calculated from Kaplan-Meier estimates.