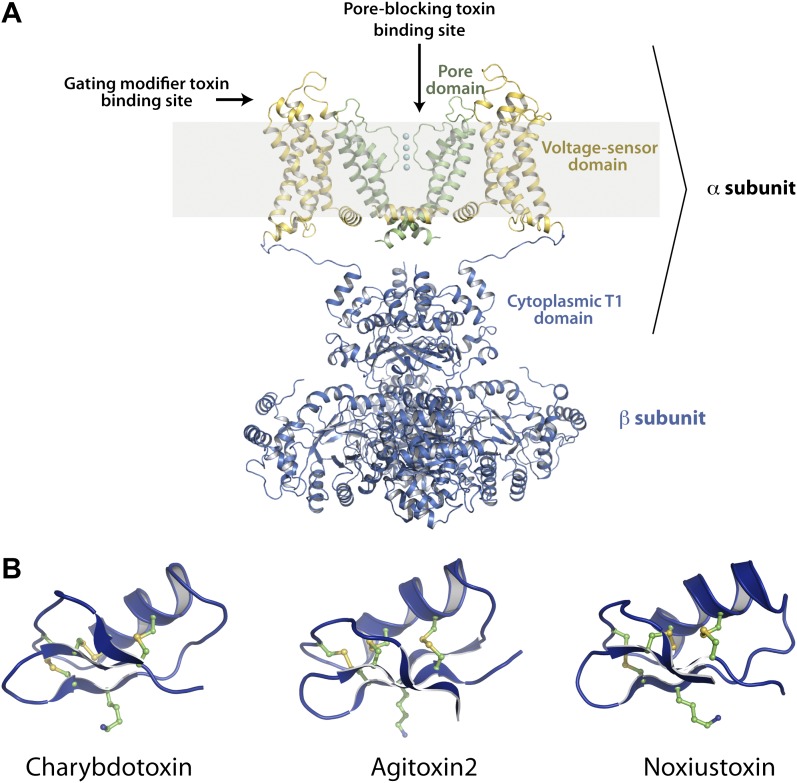
Figure 1. Structures of the channel used in this study and of representative scorpion toxins, including the one used in this study.
(A) Side view showing two diagonal subunits of the paddle chimera channel in complex with the auxiliary β-subunit shown in ribbon trace (PDB ID 2R9R; Long et al., 2007). The pore domain of paddle chimera is colored green, and the voltage sensor domain and the linker between the voltage sensor and the pore are colored in yellow. The cytoplasmic T1 domain and the auxiliary β-subunit are shown in blue. The K+ ions in the selectivity filter are shown as cyan spheres. The area corresponding to the membrane is shaded in light gray. The channel forming α-subunit is indicated. Each α-subunit forms a complex with a β-subunit and four such α- and β-heterodimers make up the tetramer. Note that because the voltage sensors arrange around the pore domains in a domain-swapped fashion, the voltage sensor domains and the pore domains shown in the figure belong to different molecules of the tetrameric channel. Sites on the channel for binding the pore-blocking toxins and gating-modifier toxins are shown with arrows. (B) The lowest energy NMR structures of representative scorpion toxins with activity on Kv channels—charybdotoxin (CTX; PDB ID 2CRD; Bontems et al., 1991), Agitoxin2 (AgTx2; PDB ID 1AGT; Krezel et al., 1995) and Noxiustoxin (PDB ID 1SXM; Dauplais et al., 1995) are shown in blue ribbon trace. A critical lysine (Lys27 for CTX and AgTx2 and Lys28 for Noxiustoxin) that is conserved in this family of toxins and the conserved cysteines are shown in ball and stick rendition and are colored by atoms.