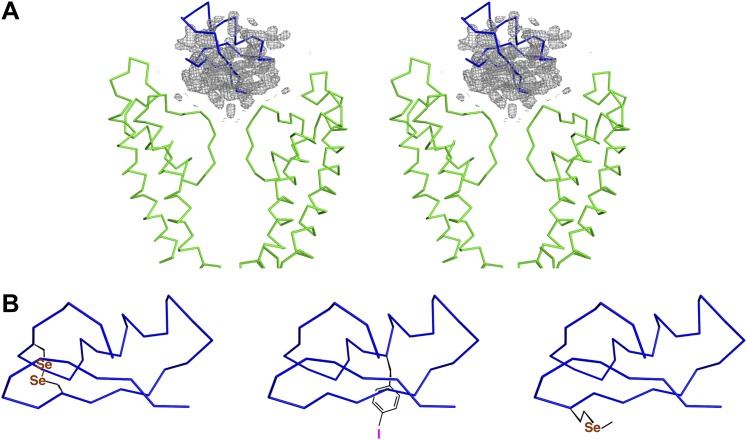
Figure 4. Initial placement of the CTX molecule in the toxin-channel complex and heavy atom derivatives used for subsequently improving the accuracy of placement.
(A) Stereoview showing the pore domains from two diagonal subunits (molecule A) of paddle chimera (in the toxin-channel complex) in green α carbon trace and a toxin-omit weighted 2Fo − Fc electron density map in wire mesh at 0.8 σ contour level. The initial placement of the CTX molecule using the NMR structure (PDB ID 2CRD; Bontems et al., 1991) is shown in blue α carbon trace within the omit map. (B) Chemical structures of the heavy atom derivatives of CTX used in this study are shown schematically. The NMR structure (PDB ID 2CRD; Bontems et al., 1991) is used to depict the rest of the molecule in blue α carbon trace. The heavy atoms are shown in color, purple for iodine and copper for selenium.