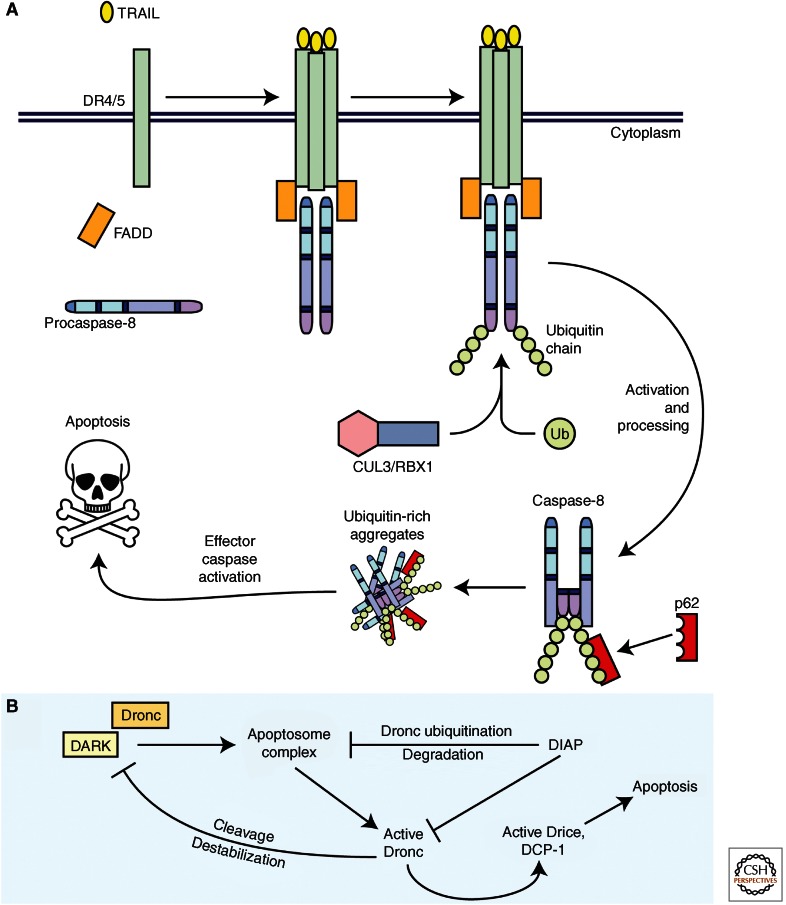
Figure 4.
Novel mechanisms of ubiquitylation in caspase regulation. (A) A novel mechanism in which caspase-8 activity is positively regulated via ubiquitylation. A proapoptotic ligand, such as TRAIL, initiates the extrinsic apoptotic pathway at the plasma membrane through formation of the DISC and recruitment of procaspase-8. Caspase-8 is then activated through induced-proximity dimerization. Once active, caspase-8 can be ubiquitylated by the neddylated form of CUL3/RBX1. This posttranslational modification on caspase-8 allows for its interaction with p62. Through autoprocessing, active caspase-8 releases the catalytic domains, which remain bound to p62. This active caspase-8 is moved into cytosolic aggregates rich in ubiquitin in which the caspase-8 remains active through stabilization of the dimer. (B) A unique mechanism for a negative feedback loop between DARK (dApaf-1) and Dronc. The Drosophila IAP, DIAP1, inhibits Dronc and Drice/DCP-1 by interacting with the caspases. DIAP1 also indirectly reduces downstream effector caspase activity by facilitating degradation of the apoptosome complex through ubiquitylation of DARK-bound active Dronc. Active Dronc can also negatively feedback on DARK by directly cleaving the protein, leading to destabilization of DARK and reduced protein levels. Thus, Dronc and DARK are involved in a DIAP1-dependent negative feedback loop.