Abstract
Background
The main hurdles to the wide spread use of islet transplantation for the treatment of type 1 diabetes continue to be the insufficient number of appropriate donors and the need for immunosuppression. Microencapsulation has been proposed as a means to protect transplanted islets from the host’s immune system.
Methods
The present study investigated the function of human pancreatic islets encapsulated in Ca2+/Ba2+-alginate microbeads intraperitoneally transplanted in diabetic Balb/c mice.
Results
All mice transplanted with encapsulated human islets (n=29), at a quantity of 3,000 islet equivalent (IEQ), achieved normoglycemia one day after transplantation and retained normoglycemia for extended periods of time (mean graft survival 134 ± 17 days). In comparison, diabetic Balb/c mice transplanted with an equal amount of non-encapsulated human islets rejected the islets within 2–7 days after transplantation (n=5). Microbeads retrieved after 232 days (n=3) were found with little to no fibrotic overgrowth and contained viable insulin positive islets. Immunofluorescent staining on the retrieved microbeads showed F4/80 positive macrophages and alpha SMA positive fibroblasts but no CD3 positive T lymphocytes.
Conclusions
The Ca2+/Ba2+-alginate microbeads can protect human islets from xenogeneic rejection in immunocompetent mice without immunosuppression. However, grafts ultimately failed likely secondary to a macrophage mediated foreign body reaction.
Keywords: Human islets, diabetes, transplantation, alginate, microcapsule, microbead
Introduction
Clinical islet transplantation has dramatically improved the quality of life for many insulin-dependent diabetic patients, but it has been hindered by the need for immunosuppression and an insufficient number of donors (1–3). Microencapsulation of islet cells potentially provides a means to eliminate immunosuppression and use xenogeneic tissue for transplantation (4–8).
Since the first evidence that microencapsulated islets could regulate blood glucose in rats in 1980 (9), a variety of semi-permeable membranes have been evaluated. Alginate has been the most frequently used material for encapsulation (4). Alginate produced in brown algae and some bacteria is a linear polyanion consisting of α-L-gluluronic acid (G) and β-D-mannuronic acid (M). Coating of the alginate microbeads with a polycation (e.g. poly-L-lysine (PLL) or poly-L-ornithine (PLO)) increases the stability and reduces the permeability to block antibodies from entering the microcapsules (10–12). However, as polycations are known to cause cell adhesion (13) and are shown to induce inflammation through TNF-production and necrosis (14, 15), strategies for omitting the polycation layer have been developed.
For stabilization of the microbeads without a polycation, Ba2+ has frequently been used to crosslink alginate as it is known to strongly bind to alginate with a high content of G (12). Although alginate microbeads exhibit a higher permeability than alginate-polycation microcapsules, Ba-alginate microbeads have been shown to protect porcine islets upon transplantation to rodents and large animals (16–20). Indeed, Safley and coworkers (21) reported longer graft survival for xenografts in Ba-alginate microbeads than in alginate-PLL microcapsules. However, as barium leakage from microbeads raises safety concerns (22, 23), we decided to explore the capability of alginate microbeads crosslinked by a minimal amount of barium in a xeno-model.
We have previously shown that microbeads made of alginate with a high content of G, in combination with 50 mM of calcium and 1 mM of barium, are stable against osmotic swelling. However, they have greater permeability compared to using barium as the only crosslinking ion (12). Human islets encapsulated in these Ca2+/Ba2+-alginate microbeads can function in immunodeficient mice for approximately 300 days (24). Recently, a study using an anti-coagulated whole blood model showed that the Ca2+/Ba2+-alginate and Ba2+-alginate microbeads are not inducing complement activation in contrast to other microcapsule types in contact with human blood (13). The purpose of the current study was to evaluate the immunoprotective function of the Ca2+/Ba2+-alginate microbeads in a xeno-transplant model in diabetic mice using human islets.
Materials and Methods
Encapsulation of human islets in Ca2+/Ba2+-alginate microbeads
Human islets were harvested from heart-beating deceased organ donors according to the standardized University of Illinois at Chicago (UIC) protocol (25, 26). The microbead production was performed as previously described (24). The islet suspension was centrifuged (1,000 rpm for 2 min at RT), resuspended in a small volume of culture media (CMRL-1066), and mixed with a 2.0% (w/v) sterile-filtered alginate solution (NovaMatrix, Sandvika, Norway two batches of alginates were used with the following specification: Protanal LF10/60 63% G, MW 200 kDa, endotoxin < 1000 EU/g; UP-MVG 67% G, MW 240 kDa, endotoxin < 43 EU/g) in 0.3 M mannitol (pH 7.2–7.4) to a final suspension of 10,000 IEQ per ml of 1.8% (w/v) alginate solution. The alginate-islet suspension was dripped into a gelling solution of 50 mM CaCl2 and 1 mM BaCl2 (in 10 mM MOPS, 0.14 M mannitol and 0.05% Tween 20; pH 7.2–7.4), over a period of 15 minutes, to form microbeads. At this point, the microbeads were left in the gelling bath for another 7 minutes to ensure that the gelling reached the saturation level. For the dripping procedure, an electrostatic microbead generator (7 kV, flow 10 ml/hr per 0.4 mm needle) was used, resulting in alginate microbeads of 480 – 550 µm in diameter. The alginate microbeads were collected on a filter and washed three times with approximately 20 ml of culture media over a period of 5 minutes. The encapsulated islets were then transferred to Petri dishes for culture, using CMRL-1066 culture media supplemented with 2 mM glutamine (Invitrogen) and Pennicillin/Streptomycin (Invitrogen), at 37°C and 5% CO2. All encapsulation and culture procedures were performed under sterile conditions. A total of five batches of encapsulated islets were made for transplantation.
Viability and static glucose incubation tests were performed on the islets as previously described (24) before and after encapsulation to make sure the encapsulation process did not alter islet function.
Measurement of permeability of Ca2+/Ba2+ -alginate microbeads
Two techniques were used to assess the permeability of the empty microbeads. The first technique was based on the quantification of fluorescently labeled IgG (Alexa Fluo 488 goat anti-human IgG; Molecular Probes) that permeated inside the alginate microbeads in 24 hours by confocal laser scanning microscopy (CLSM). The amount of permeated IgG was calculated as a percentage of the mean fluorescence intensity of labeled solute inside the microbead compared to the mean fluorescence intensity detected outside the microbead (Iinside/Ioutside * 100).
The second technique used to assess microbead permeability, as a complementary technique to CLSM, was inverse size exclusion chromatography, which provides a molecular weight cut-off (MWCO) value for the tested microbeads. This value is determined based on the exclusion limit of the chromatography column made of the tested microbeads (27, 28). The partition coefficient (KSEC) was calculated from the elution volumes corresponding to 50% of the area of saccharose and narrow distributed pullulan standards (Polymer Laboratories Ltd) with molecular weights between 667 and 805 000 g/mol injected as solutes of known molecular weight to the column. KSEC plotted against the molecular weight of standards results in the calibration curve of the column made of microbeads, from which the MWCO value is determined as the exclusion limit of the column (28).
SEM analysis of naked islets and Ca2+/Ba2+-alginate microbeads with islets
Scanning Electron Microscopy (SEM) was used to image the surface of islets and microbeads before transplantation. Islets and encapsulated islets were fixed in 2.5% glutaraldehyde (Sigma, St. Louis, MO) in HBSS and shipped to the University of Alberta, Canada. Microbeads were then pre-fixed in 2.5% glutaraldehyde in Millonig’s buffer (pH 7.2) at room temperature for 1 hour, post fixed in 1% osmium tetraoxide for 1 hour, and dehydrated through an ascending series of ethanol concentrations up to 100%. They were critical point-dried, mounted, and sputter-coated with gold. Micrographs were taken with a Hitachi Electron Microscope S-2500.
Transplantation of encapsulated human islets
A total of 34 male immunocompetent Balb/c mice (Harlan Industries; Indianapolis, IN), weighing 22–25 g, were used as recipients for both encapsulated and non-encapsulated human islets. Diabetes was induced by intraperitoneal (IP) injection of Streptozotocin (STZ, 180 mg/kg body weight; Sigma, St. Louis, MO) freshly dissolved in citrate buffer (pH 4.5). Animals were considered diabetic after the measurement of non-fasting blood glucose levels, sampled from the tail vein, exceeded 350 mg/dl for two consecutive days. Diabetes generally occurs within a time period of 72 hours post STZ injection. Twenty-nine diabetic mice were used as recipients for encapsulated islets and five mice were transplanted with an equal amount of non-encapsulated islets. All recipient mice underwent intraperitoneal transplantation.
On the day of transplant, the encapsulated or non-encapsulated human islets were collected and washed 5 times with 250 ml of Hank’s buffer salt solution (HBSS) in order to remove xenogeneic components from the media. The transplantation procedure was conducted in a biological safety cabinet under sterile conditions. Recipient mice were first placed under gas anesthesia (isoflurane) using a mini-mask (Viking Medical). A short midline abdominal incision (approximately 0.5 cm) was made and 3,000 IEQ of either the encapsulated or non-encapsulated islets were slowly infused into the peritoneal cavity using a 2 ml Pasteur pipette. Blood glucose and body weight were monitored every other day for the first week and then twice a week until the end of the study. Graft failure was defined as the measurement blood glucose levels exceeding 210 mg/dl for two consecutive days. All of the diabetes induction and transplant procedures were performed under the guidelines of the National Institutes of Health and approved by the Animal Care Committee at UIC.
Oral Glucose Tolerance Test (OGTT)
An OGTT was performed five weeks after transplantation in mice with encapsulated islets (n=5), non-transplanted diabetic mice (n=5), and normal control mice (n=5) according to the method described by Korbutt et al with some modifications (24, 29). Briefly, after 16–18 hours of fasting, D-glucose (3 g/kg body weight) was administered as a 20% solution by gastric gavage using PE 50 polyethylene tubing (Becton Dickinson, Sparks, MD) into anaesthetized mice (isoflurane). Blood glucose levels, sampled via tail puncture, were recorded at 0, 15, 30, 60, 90, and 120 min.
Retrieval of microbeads and fibrosis scoring
Microbeads were retrieved from three mice 232 days after transplantation. Briefly, under gas anesthesia (isoflurane), a midline abdominal incision (approximately 0.5 cm) was made and 3 ml of warmed sterile HBSS containing 2mM CaCl2 was infused into the peritoneal cavity to wash out the microbeads. This procedure was repeated five times, and then the animals were cut open to look for possible adhesions and clusters of microbeads. During the washing process, in addition to the microbeads, tissue clusters were also collected from the peritoneal cavity. The harvested microbeads and tissue clusters were collected in culture medium for further analysis. The degree of pericapsular overgrowth of the microbeads was determined by a method similar to that described by Wilson et al (30). In brief, retrieved microbeads were assigned a score ranging from 0 to 4 based on approximate percentage of the microbeads’ surface covered by cells: 0 was defined as no cellular overgrowth and 4 was defined as 100% cellular overgrowth. Scores of 1, 2 and 3 corresponded to approximately 25, 50 and 75 %, respectively, of surface cellular overgrowth. A total number of 60 microbeads were assessed by this method for each animal.
SEM and immunostaining of retrieved microbeads
SEM analysis on the retrieved microbeads was conducted using previously described methods. For histological assessment, the encapsulated islets were fixed in Bouin’s solution, suspended in 2% agarose gel, and embedded in paraffin. Five µm sections of the paraffin embedded tissue were mounted on poly-L-lysine coated slides and serial sections were stained for Hematoxyllin-Eosin (HE), insulin, and immune cell infiltration (mouse macrophage marker F4/80, tissue derivative macrophage marker CD68, fibroblast marker alpha-smooth muscle actin (α-SMA), and T cell marker CD3). All images were viewed on a Leica DM 2000 microscope and captured via QICAM fast1394 and Qcapture Pro 5.1 programs.
Statistical Methods
In 5 separate experiments of transplantation, 2 batches of alginate and 5 islet donors were used. The data were pooled as no statistically significant difference was found between the two batches of alginate. Results were expressed as mean ± SD. Multiple comparisons were analyzed via t-test or ANOVA where applicable. The log-rank test was used to compare graft survival for encapsulated vs. non-encapsulated islets transplanted to diabetic mice in Kaplan Meier survival curves. A P value < 0.05 was considered statistically significant.
Results
Encapsulation outcomes
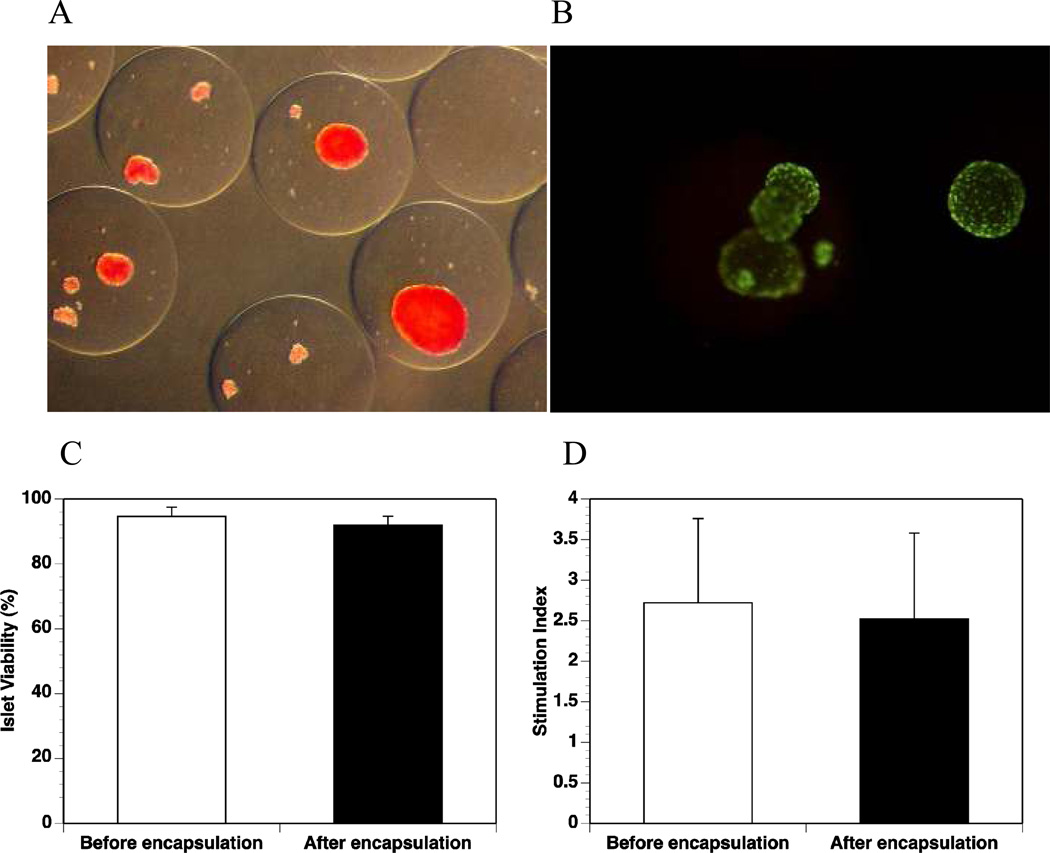
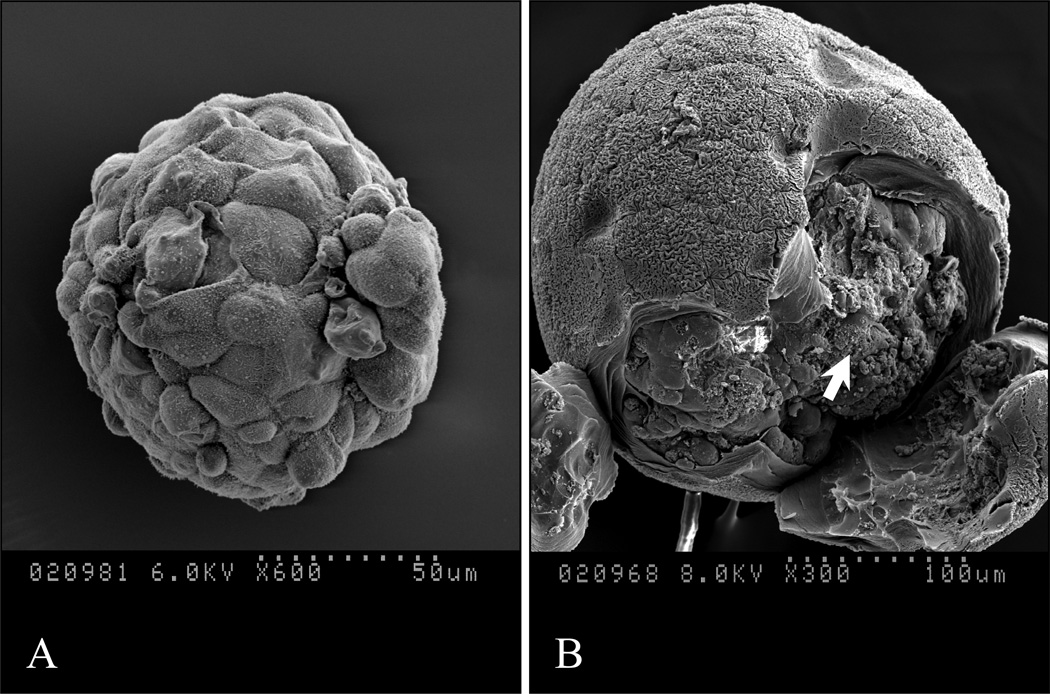
After encapsulation in the Ca2+/Ba2+-alginate microbeads, the islets maintained their morphology as determined by staining with dithizone (Fig. 1A). The viability (Fig. 1B and C) and insulin secretion response upon high glucose stimulation (Fig. 1D) were well maintained. There were no significant differences in terms of viability (before encapsulation vs. after encapsulation: 94.6 ± 2.9% vs. 92.0 ± 2.7%, p=0.09) and functionality as measured by insulin secretion upon glucose stimulation (stimulation index before encapsulation vs. after encapsulation: 2.72 ± 1.04 vs. 2.52 ± 1.06, p=0.38). Scanning electron micrographs of non-encapsulated islet and islet in a microbead that was cut open for visualization of the islet are shown in Fig. 2 (the microbeads shrank to about 50% of their original size during processing before SEM).
Figure 1. Microencapsulation of human islets in Ca2+/Ba2+-alginate beads.
(A): Dithizone staining of the encapsulated islets; (B): Viability staining (Sytogreen, green for alive cells; Ethidium bromide, red for dead cells) of the encapsulated islets; (C): Viability values of the islets before and after encapsulation (n=5, p=0.09); (D): Stimulation index values of islets before and after encapsulation (n=5, p=0.38).
Figure 2. Scanning electron microscopy (SEM).
(A): Non-encapsulated islet before transplantation; (B): Encapsulated islet before transplantation (the islet is indicated by the arrow).
Permeability of the Ca2+/Ba2+ alginate microbeads
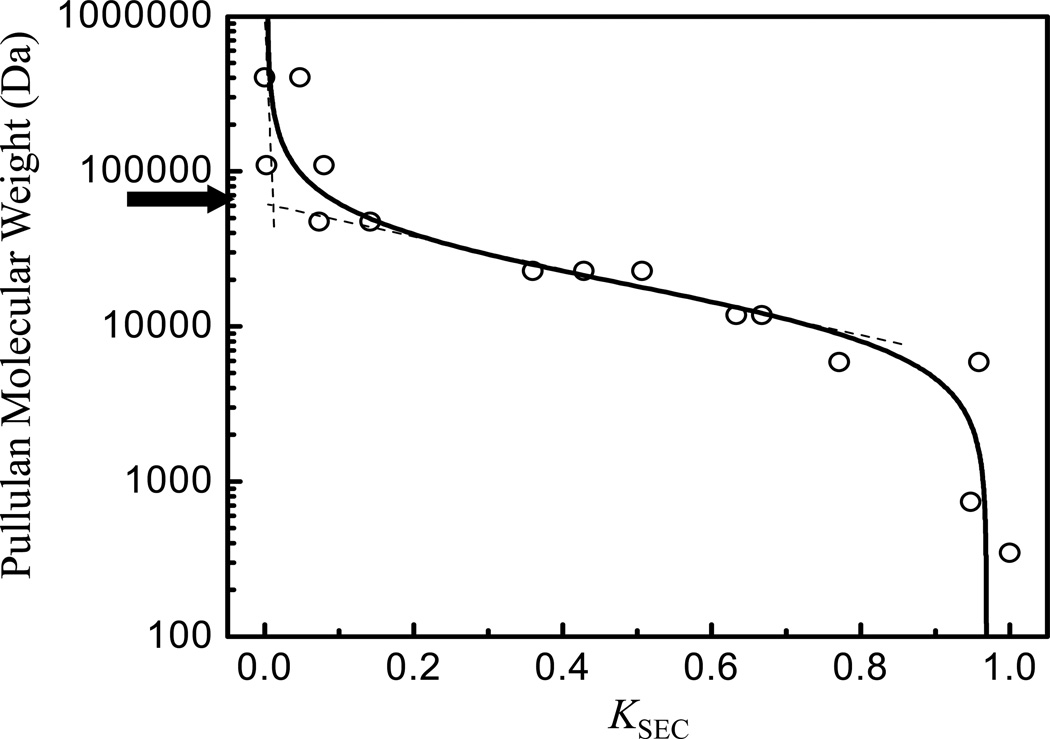
From the inverse size exclusion chromatography, a MWCO value of 60 kDa with regard to pullulan standards was found (Fig. 3), which can be recalculated to corresponding viscosity diameters, following the work of Brissova et al. (27), leading approximately to the value of 12 nm. This value of viscosity diameter can be used to estimate the MWCO value of microbeads in terms of globular proteins (27) and equals to ~ 350 kDa, which is a much higher value than the molecular weight of IgG (150 kDa). In agreement with this estimation of permeability of microbeads by inverse size exclusion chromatography, the IgG permeation to microbeads was confirmed by CLSM when about 45 % of mean fluorescence intensity of fluorescently labeled IgG was found after 24 hours compared to that of the outside solution.
Figure 3. Permeability measurement of alginate microbeads.
Determination of the molecular weight cut-off (MWCO = 60 kDa, as indicated by an arrow) for alginate microbeads by an inverse size exclusion chromatography from dependence of molecular weight of pullulan standards on their corresponding partition coefficient KSEC. Circles are experimental data and the line is the best fit of data used to determine the MWCO value from its linear parts as indicated by dashed lines.
Transplantation outcome
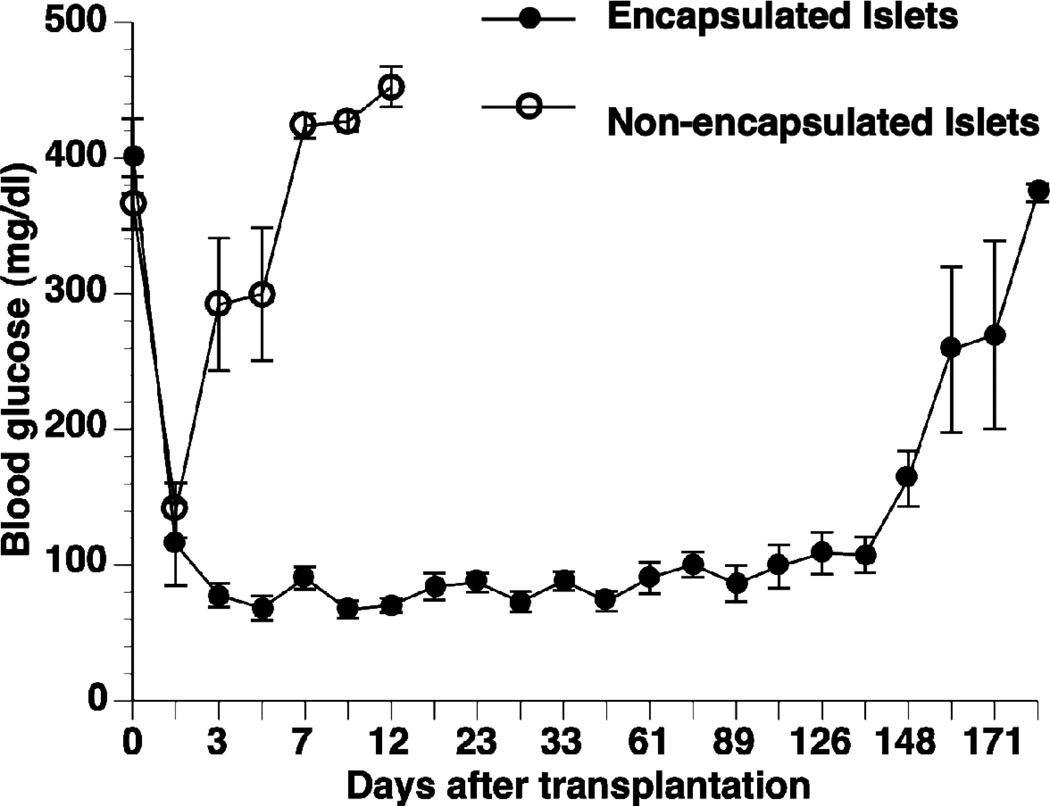
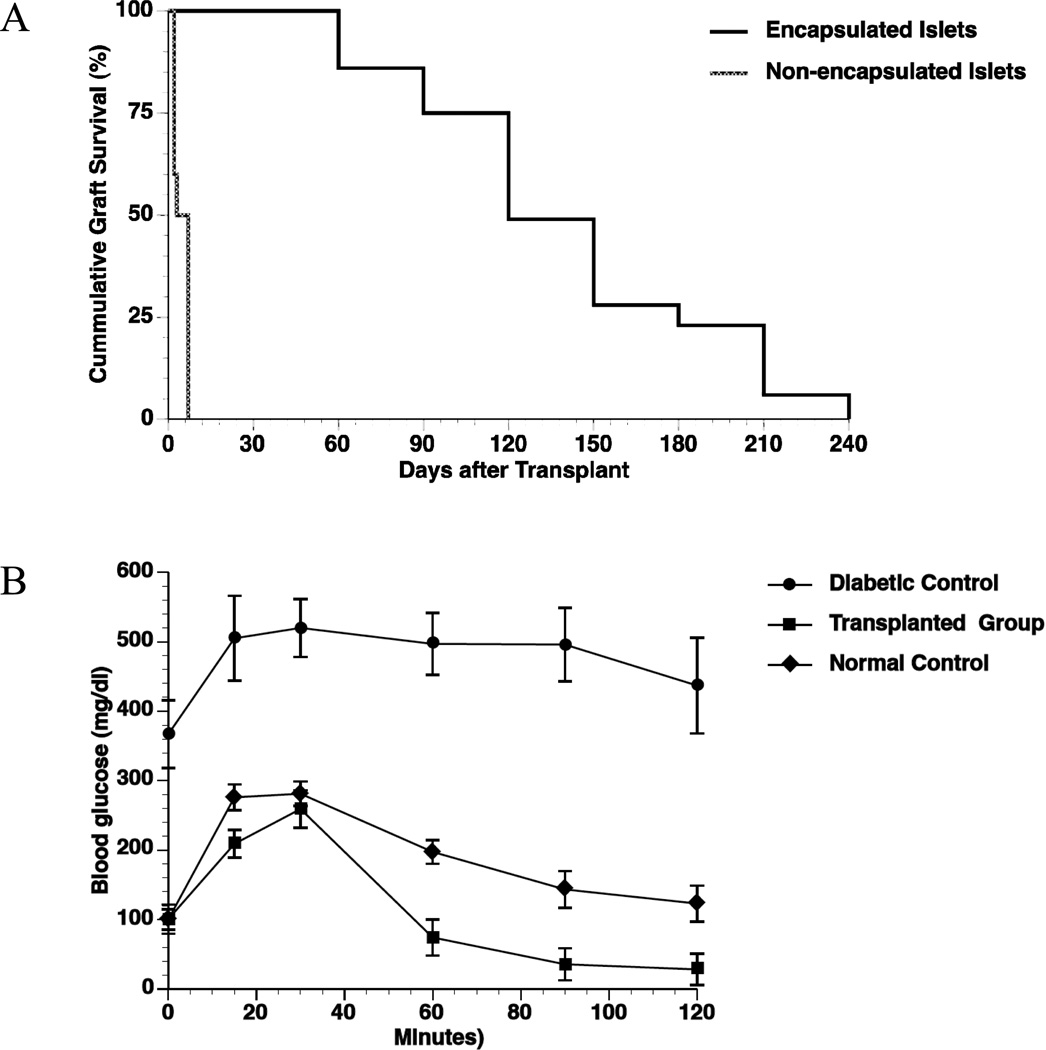
Following transplantation with encapsulated human islets, all diabetic Balb/c mice displayed normalized blood glucose levels at day 1 of post transplantation with an average graft survival time of 134 ± 17 days (n = 29). By comparison, all the diabetic mice transplanted with non-encapsulated human islets experienced graft rejection within 2–7 days (n = 5) (Fig 4). Cumulative survival of the non-encapsulated and encapsulated islet grafts is given in Fig. 5A (p<0.0001). OGTT results five weeks post transplantation showed that the mice receiving encapsulated human islets responded to high glucose stimulation similar to normal mice (Fig. 5B). The diabetic control mice maintained high blood glucose levels throughout the test, and had a significantly larger area under the curve (AUC, 58400 ± 6200) than both the normal mice (23300 ± 2500, p <0.05) and the mice receiving encapsulated human islets (13500 ± 2900, p <0.001).
Figure 4. Glycemic levels of the transplanted mice.
Blood glucose levels of the Balb/c mice transplanted with 3,000 IEQ non-encapsulated (n=5, open circle) and encapsulated human islets (n=29, close circle).
Figure 5. Cumulative graft survival curves and OGTT.
(A): Graft survival in diabetic Balb/c mice transplanted with encapsulated human islets (n=29) vs. non-encapsulated human islets (n=5); *p <0.0001; (B): Average glycemic levels during OGTT in diabetic Balb/c mice transplanted with encapsulated human islets (transplanted group, n=5), non-transplanted diabetic Balb/c mice (diabetic control, n=5), and non-transplanted, non-diabetic Balb/c mice (normal control, n=5) five weeks post transplantation.
Assessment of retrieved microbeads
Three mice receiving encapsulated human islets were sacrificed at day 232 for microbead retrieval; one mouse was normoglycemic and two mice were hyperglycemic. All explanted microbeads could be easily washed out of the peritoneal cavity, with modest or no sign of fibrosis around the microbeads (Table 1). Careful examination of the peritoneal cavity did not reveal clumps or attached microbeads. With respect to the degree of pericapsular overgrowth, 67% of the retrieved microbeads were categorized with a score of 0 (0% cellular overgrowth) in the normoglycemic mouse (Table 1). However, in the two hyperglycemic mice, the percentage of microbeads categorized with a score of 0 was only 4% and 37%, respectively.
Table.
Condition and degree of fibrosis of retrieved microbeads containing human islets from Balb/c mice 232 days post transplantation.
| Animals | Glycemic condition |
Microbead in the peritoneal cavity |
Rate of intact microbead (%) |
Score for fibrosis* (%) |
|---|---|---|---|---|
| Mouse 1 | Normoglycemia | FF | 100 | Degree 0 (67) |
| Degree 1 (15) | ||||
| Degree 2 (5) | ||||
| Degree 3 (3) | ||||
| Degree 4 (10) | ||||
| Mouse 2 | Hyperglycemia | FF | 100 | Degree 0 (4) |
| Degree 1 (4) | ||||
| Degree 2 (0) | ||||
| Degree 3 (8) | ||||
| Degree 4 (84) | ||||
| Mouse 3 | Hyperglycemia | FF | 100 | Degree 0 (37) |
| Degree 1 (21) | ||||
| Degree 2 (20) | ||||
| Degree 3 (6) | ||||
| Degree 4 (16) |
FF, Free floating
The numerical category of the fibrotic surface cellular overgrowth (0–4) corresponds to 0%, 25%, 50%, 75%, and 100%, respectively.
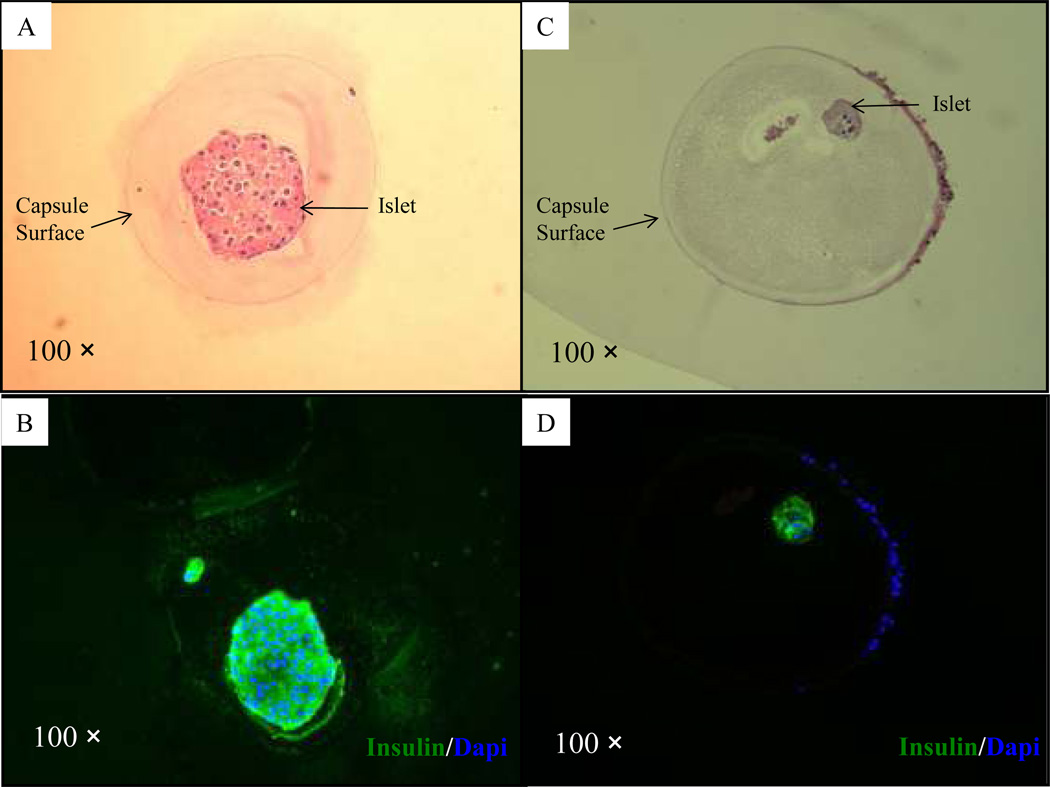
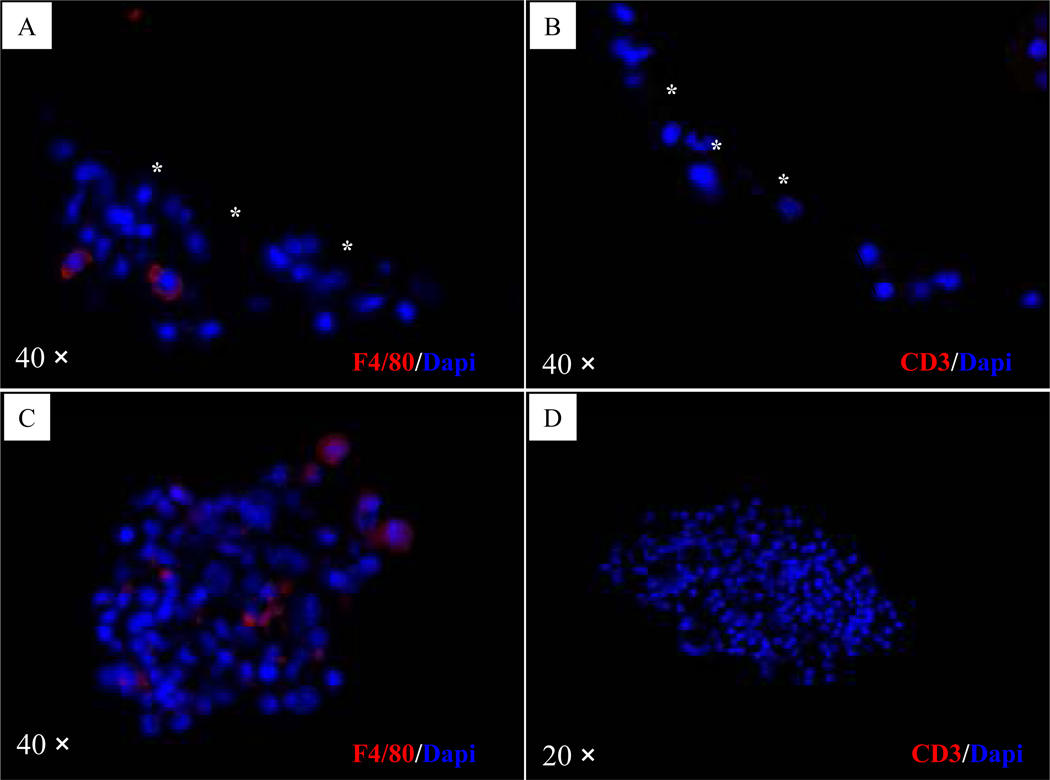
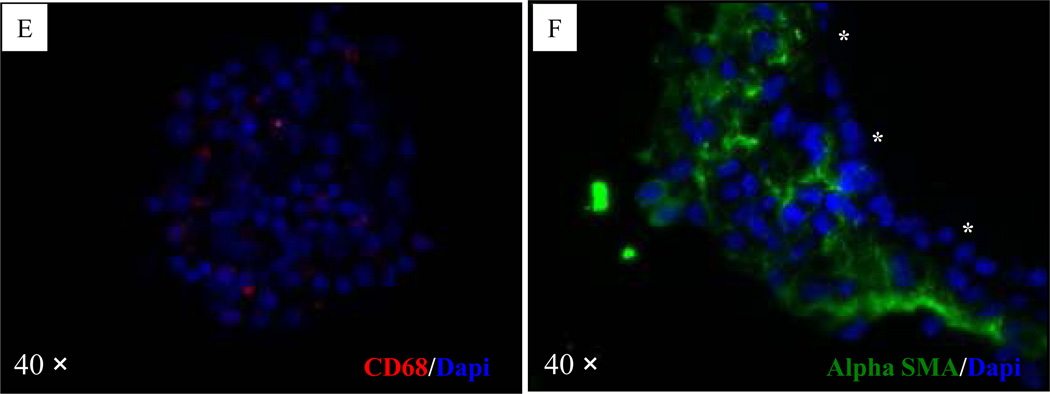
H&E and immunofluorescent staining of the microbeads from the normoglycemic mouse revealed the presence of healthy and insulin-positive islets in the microbeads free of cellular overgrowth (Fig. 6A–B). H&E and immunofluorescent staining of the microbeads from one of the hyperglyemic mice (mouse 2) revealed the presence of degenerated islets in the microbeads with cellular overgrowth (Fig. 6C–D). F4/80 positive cells were detected both in retrieved microbeads and tissue clusters in the peritoneal cavity (Fig. 7A & 7C), but CD3 positive cells were not detected in the same set of tissue blocks (Fig. 7B & 7D). The CD68 positive cells (tissue derived macrophages) were observed in tissue clusters collected in the peritoneal cavity but rarely detected on the surface of retrieved microbeads (Fig. 7E). Alpha-SMA, staining indicated that the cells in the outer layer, rather than the cells close to the surface of the retrieved microbeads, stained positive for α-SMA (Fig. 7F).
Figure 6. Immunohistochemical analysis of microbeads retrieved at day 232 after transplantation with encapsulated human islets.
(A–B): H&E staining (A) and immunofluorescent staining (B; green = insulin and blue = fluorescent DNA dye, Dapi) of a microbead retrieved from a normoglycemic mouse (mouse 1, Table 1); (C–D): H&E staining (C) and immunofluorescent staining (D) of a microbead retrieved from a hyperglycemic mouse (mouse 2, Table 1).
Figure 7. Immunohistochemical staining of retrieved microbeads and tissue clusters in the peritoneal cavity in a hyperglycemic mouse (mouse 2, Table 1).
(A): Microbeads stained for mouse macrophage marker F4/80; (B): Tissue clusters in the peritoneal cavity stained negatively for mouse macrophage marker F4/80; (C): Microbeads stained for T lymphocyte specific marker CD3; (D): Tissue clusters in the peritoneal cavity stained negatively for T lymphocyte specific marker CD3. (E): The CD68 positive cells (tissue derivative macrophages) were observed in tissue clusters collected in the peritoneal cavity. (F): The cells in the outer layer, rather than the cells close to the surface of the retrieved microbeads, stained positive for α-SMA. In the pictures A, B, and F, the overgrowth/microbead interface is marked by three asterisks.
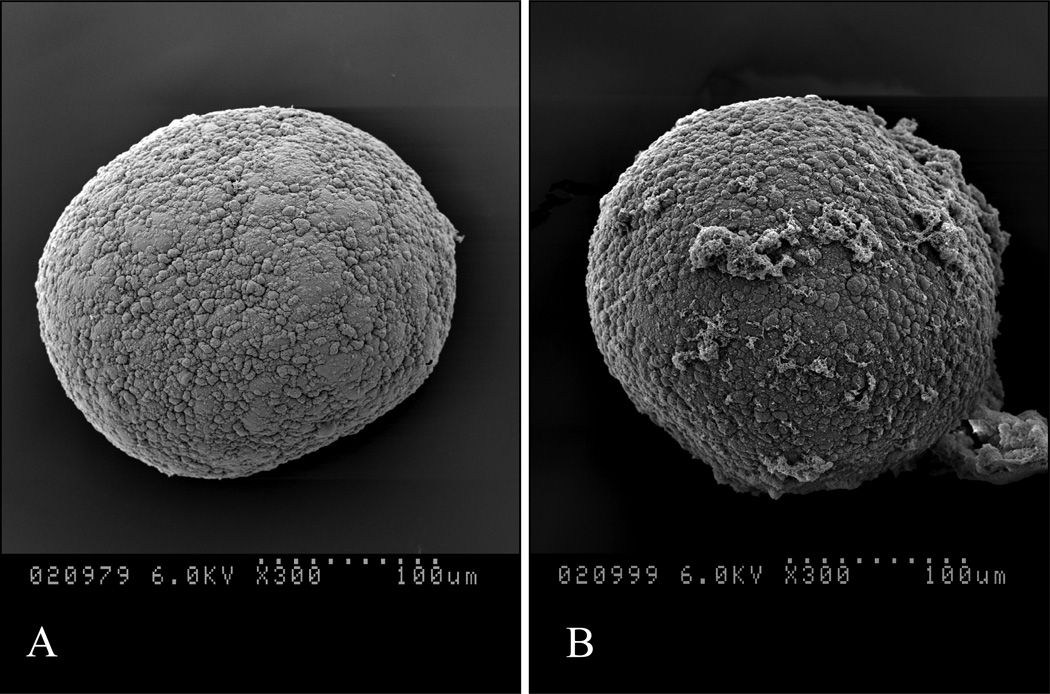
SEM pictures exemplify the microstructure of the retrieved microbeads from the normoglycemic mouse with no to minimal cell overgrowth on the microbead surface (Fig. 8A and B, respectively).
Figure 8. Scanning electron microscopy (SEM).
Retrieved microbeads at day 232 post-transplantation in the normoglycemic mouse with no (A) and minor (B) fibrotic overgrowth.
Discussion
The results from this study confirm that Ca2+/Ba2+-alginate microbeads can be reproducibly formed to encapsulate human islets that retain their function and restore normoglycemia in immunocompetent diabetic mice without the use of immunosuppression. This is the first report to demonstrate the suitability of alginate microbeads crosslinked predominantly with Ca2+ to reverse diabetes in such a stringent islet transplant model.
In the current study, we used a high-G alginate with 50 mM Ca2+ and 1 mM Ba2+ in the gelling solution. We have previously shown that the addition of 1mM of barium ions to a gelling solution of calcium dramatically improves the microbead stability (12). Further increasing the barium ion concentration in the gelling solution to 20 mM does not significantly impact the stability of the high-G alginate microbeads, although an increase in gel strength is observed together with reduced permeability towards IgG (12). In this study, the Ca2+/Ba2+-microbeads were shown to have a molecular weight cut-off with respect to globular proteins of approximately 350 kDa, and indeed were shown to be permeable to IgG. Since Ba-alginate microbeads of both high and low G content have been found to protect xenografts (16, 21), we intended to explore the immune protective properties of the open gel matrix of the Ca2+/Ba2+-alginate gel. Also, a lower concentration of Ba2+ is beneficial in reducing the leakage of barium ions from the microbeads, as described recently (22).
Transplantation studies showed that mice receiving islets in the Ca2+/Ba2+-microbeads had significantly improved graft function compared to mice receiving non-encapsulated islets. While the Ca2+/Ba2+-alginate microbeads permitted the graft survival to be significantly extended from less than one week for non-encapsulated islets to over three months for encapsulated islets, graft failure was ultimately observed in the majority of mice transplanted with encapsulated human islets with a cumulative graft survival of 6% at 210 days. OGTT analysis showed mice transplanted with encapsulated islets responded more promptly to glucose stimulation than the normal control mice. A similar OGTT pattern was observed in our previous study in nude mice transplanted with encapsulated human islets using the same alginate microbeads (24), where we suggested that the lower range of normal blood glucose in humans than in mice may be the cause of the difference between the mice islets (control) and human islets. However, a delay in glucose sensing by the prolonged diffusion distance incurred by the microbead may contribute to the occurrence of hypoglycemia after a glucose challenge.
The cause of ultimate graft failure in the majority of transplanted mice is not known. Survival of encapsulated pancreatic islets in the peritoneal cavity has been reported to vary from one week to up to more than a year, depending on the microcapsule type, recipient model, and donor tissue. The oxygen consumption rate, geometry of the microcapsule, tissue density, and spatial arrangement of the encapsulated tissue have been considered as critical factors that can affect oxygen supply to the encapsulated tissue (31). In this study, all mice were transplanted with 3,000 IEQ encapsulated human islets, the same dose of islet in the same microbead type for which we have previously seen graft survival times of more than 300 days in an immune-compromised nude mice model (24). This indicates that a lack of oxygen supply by itself may not be the main reason for graft failure. The late stage graft failure observed in this study could be the result of a late stage immune rejection and overgrowth of the microbeads.
Previous studies on microencapsulated xenografts in mice have mainly used porcine islets (16, 32). For adult porcine islets (API) transplanted to nonobese diabetic (NOD) mice, Safley and coworkers (21) reported longer graft survival for xenografts in alginate microbeads than in alginate-PLL microcapsules. Alginate microbeads are normally considered to have an increased permeability compared to alginate-PLL microcapsules, hence for a xenograft one would hypothesize better results for the alginate-PLL microcapsule. The survival of encapsulated API in NOD mice has been further prolonged to more than 450 days by a costimulatory blockade (32).
The histological study on the retrieved microbeads revealed that macrophages but not specific T lymphocyte cells dominated the stimulated immune cell population after transplantation of microencapsulated islets. Those results suggest that the response is a non-specific, innate-immune system mediated inflammatory response rather than a specific T-cell-mediated one.
The numbers of retrieved microbeads without fibrosis in this study varies from 67 % for the normoglycemic mouse to 4 and 37 % for the hyperglycemic mice (Table 1). We have previously shown that empty alginate beads implanted intraperitoneally in Balb/c mice resulted in 91 ± 6% microbeads without fibrosis after 40 days (15) with exactly the same alginate as used in the current study with the endotoxin content of < 1000 EU/g For encapsulated porcine neonatal pancreatic cell clusters (NPCC), about 80% of transplanted Ba2+-alginate beads were reported to have no cell attachment 20 weeks after transplantation into B6AF1 mice (16). This indicates that the fibrotic response is higher towards microbeads containing human islets than towards empty microbeads. However for direct and more thorough comparison, a comparative study has to be made.
In summary, this study shows that Ca2+/Ba2+-alginate microbeads can protect human islets against xenograft rejection in immunocompetent diabetic mice. Although the Ca2+/Ba2+-alginate microbeads permitted the graft survival to be significantly extended from less than one week (non-encapsulated islets) to over 3 months (encapsulated islets), the graft ultimately failed likely due to cellular overgrowth and a limited fibrotic reaction. The precise mechanism leading to cellular overgrowth and graft failure over time needs to be further elucidated.
Acknowledgments
This study was conducted as part of the Chicago Diabetes Project, an international effort for a functional cure for diabetes. The study was sponsored by the Christopher Foundation, the Efroymson Fund, the Wirtz Family, and the College of Medicine at UIC. J.O. is supported by NIH (ICR Grant 1 U42 RR023245-01) and JDRF (#5-2006-398, #5-2006-424 and #5-2007-773), as well as by the Washington Square Health Foundation and the Grant Health Care Foundation. B.L.S. is supported by the Norwegian Diabetes Association/Norwegian Foundation for Health and Rehabilitation. I.L., G.K. and D.Ch. were also supported by the Slovak Research and Development Agency under contract No. APVV-0486-10.
We sincerely thank Dr. Ming H. Chen for performing the scanning electron microscopy analysis. We are grateful to Dr. Bernie Tuch and Dr. Vijay Ganapathy for kindly reviewing the manuscript.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8:1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.De Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Abalovich AG, Bacque MC, Grana D, Milei J. Pig pancreatic islet transplantation into spontaneously diabetic dogs. Transplant Proc. 2009;41:328–330. doi: 10.1016/j.transproceed.2008.08.159. [DOI] [PubMed] [Google Scholar]
- 6.Foster JL, Williams G, Williams LJ, Tuch BE. Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation. 2007;83:1440–1448. doi: 10.1097/01.tp.0000264555.46417.7d. [DOI] [PubMed] [Google Scholar]
- 7.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 8.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 9.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 10.King G, Daugulis A, Faulkner P, Goosen M. Alginate-polylysine microcapsules of controlled membrane molecular weight cutoff for mammalian cell culture engineering. Biotechnol Prog. 1987;3:231–240. [Google Scholar]
- 11.Kulseng B, Thu B, Espevik T, Skjak-Braek G. Alginate polylysine microcapsules as immune barrier: permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant. 1997;6:387–394. doi: 10.1177/096368979700600405. [DOI] [PubMed] [Google Scholar]
- 12.Mørch YA, Donati I, Strand BL, Skjåk-Bræk G. Effect of Ca2+, Ba2+ and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 13.Rokstad AM, Brekke OL, Steinkjer B, Ryan L, Kollarikova G, Strand BL, et al. Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model. Acta Biomater. 2011 Jun;7:2566–2578. doi: 10.1016/j.actbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Vandenbossche GMR, Bracke ME, Cuvelier CA, Bortier HE, Mareel MM, Remon JP. Host-reaction against alginate-polylysine microcapsules containing living cells. J Pharm Pharmacol. 1993;45:121–125. doi: 10.1111/j.2042-7158.1993.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 15.Strand BL, Ryan TL, In't Veld P, Kulseng B, Rokstad AM, Skjak-Brek G, et al. Poly-L-Lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10:263–275. [PubMed] [Google Scholar]
- 16.Omer A, Duvivier-Kali VF, Trivedi N, Wilmot K, Bonner-Weir S, Weir GC. Survival and maturation of microencapsulated porcine neonatal pancreatic cell clusters transplanted into immunocompetent diabetic mice. Diabetes. 2003;52:69–75. doi: 10.2337/diabetes.52.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Six-month survival of microencapsulated pig islets and alginate biocompatibility in primates: Proof of concept. Transplantation. 2006;81:1345–1353. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- 18.Meyer T, Hocht B, Ulrichs K. Xenogeneic islet transplantation of microencapsulated porcine islets for therapy of type I diabetes: long-term normoglycemia in STZ-diabetic rats without immunosuppression. Pediatr Surg Int. 2008;24:1375–1378. doi: 10.1007/s00383-008-2267-9. [DOI] [PubMed] [Google Scholar]
- 19.Lanza RP, Kuhtreiber WM, Ecker D, Staruk JE, Chick WL. Xenotransplantation of porcine and bovine islets without immunosuppression using uncoated alginate microspheres. Transplantation. 1995;59:1377–1384. doi: 10.1097/00007890-199505270-00003. [DOI] [PubMed] [Google Scholar]
- 20.Lanza RP, Ecker DM, Kuhtreiber WM, Marsh JP, Ringeling J, Chick WL. Transplantation of islets using microencapsulation: studies in diabetic rodents and dogs. J Mol Med. 1999;77:206–210. doi: 10.1007/s001090050337. [DOI] [PubMed] [Google Scholar]
- 21.Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J Diabetes Sci Technol. 2008;2:760–767. doi: 10.1177/193229680800200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mørch ÝA, Qi M, Gundersen PO, Formo K, Lacík I, Oberholzer J, Strand B. Binding and Leakage of Barium in Alginate Microbeads. Journal of Biomedical Materials Research: Part A. 2012;100A:2939–2947. doi: 10.1002/jbm.a.34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.EPA. Toxological review of barium and compounds. Cincinnati, OH: United States Environmental Protection Agency; 2005. p. 57. [Google Scholar]
- 24.Qi M, Strand BL, Morch Y, Lacik I, Wang Y, Salehi P, et al. Encapsulation of human islets in novel inhomogeneous alginate-ca2+/ba2+ microbeads: in vitro and in vivo function. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:403–420. doi: 10.1080/10731190802369755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi M, Barbaro B, Wang S, Wang Y, Hansen M, Oberholzer J. Human pancreatic islet isolation: Part I: digestion and collection of pancreatic tissue. J Vis Exp. 2009 doi: 10.3791/1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi M, Barbaro B, Wang S, Wang Y, Hansen M, Oberholzer J. Human pancreatic islet isolation: Part II: purification and culture of human islets. J Vis Exp. 2009 doi: 10.3791/1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brissova M, Petro M, Lacik I, Powers AC, Wang T. Evaluation of microcapsule permeability via inverse size exclusion chromatography. Anal Biochem. 1996;242:104–111. doi: 10.1006/abio.1996.0435. [DOI] [PubMed] [Google Scholar]
- 28.Brissova M, Lacik I, Powers AC, Anilkumar AV, Wang T. Control and measurement of permeability for design of microcapsule cell delivery system. J Biomed Mater Res. 1998;39:61–70. doi: 10.1002/(sici)1097-4636(199801)39:1<61::aid-jbm8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Korbutt GS, Mallett AG, Ao Z, Flashner M, Rajotte RV. Improved survival of microencapsulated islets during in vitro culture and enhanced metabolic function following transplantation. Diabetologia. 2004;47:1810–1818. doi: 10.1007/s00125-004-1531-3. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JT, Cui W, Sun XL, Tucker-Burden C, Weber CJ, Chaikof EL. In vivo biocompatibility and stability of a substrate-supported polymerizable membrane-mimetic film. Biomaterials. 2007;28:609–617. doi: 10.1016/j.biomaterials.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997;831:145–167. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 32.Cui H, Tucker-Burden C, Cauffiel SM, Barry AK, Iwakoshi NN, Weber CJ, et al. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation. 2009;88:160–169. doi: 10.1097/TP.0b013e3181abbfc1. [DOI] [PubMed] [Google Scholar]