Fig. 2.
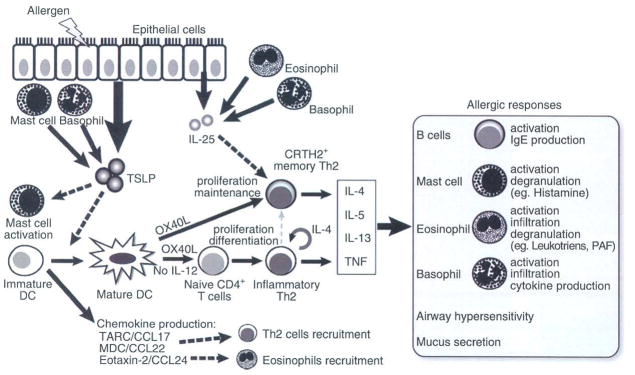
Schematic illustration of the TSLP-mediated allergic inflammation. Insults from allergens or certain pathogens trigger the aberrant expression of TSLP by epithelial cells exposed to the environment (such as skin keratinocytes and airway epithelium), mast cells and basophils. TSLP in turn activates DCs residing in the epithelium to migrate to draining lymph nodes, where they prime naïve T cells. During this process, TSLP-activated DCs (TSLP-DCs) preferentially express OX40L without IL-12 production and concomitantly produce chemokines including TARC/CCL17, MDC/CCL22, and eotaxin-2/CCL24, which function as chemoattractants for Th2 cells and eosinophils. DC-expressing OX40L strongly primes naïve CD4+ T cells to differentiate into inflammatory Th2 cells with the capacity to produce IL-4, IL-5 and IL-13 and TNF, but little or no IL-10. OX40L is the DC-derived trigger to induce the generation of such TNF++ IL-10− inflammatory Th2 cells, while IL-4 derived from the developing Th2 cells is an autocrine enhancer of the inflammatory Th2 response. Furthermore, OX40L on TSLP-DCs and IL-25 from epithelia or infiltrated eosinophils and basophils work synergistically in maintaining the memory Th2 cell response by expansion and further enhancement of Th2 cytokine production. Inflammatory Th2 cytokines eventually initiate allergic responses by activation of B cells, mast cells, eosinophils, basophils, and airway epithelium.
