Abstract
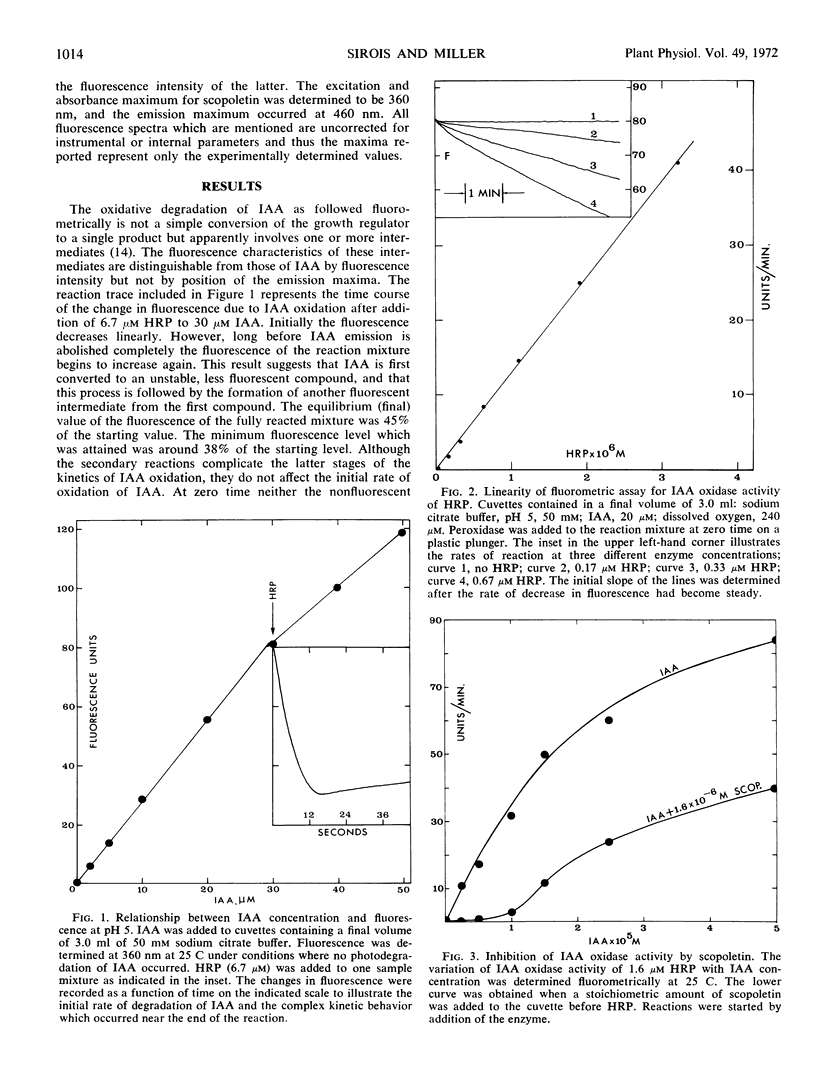
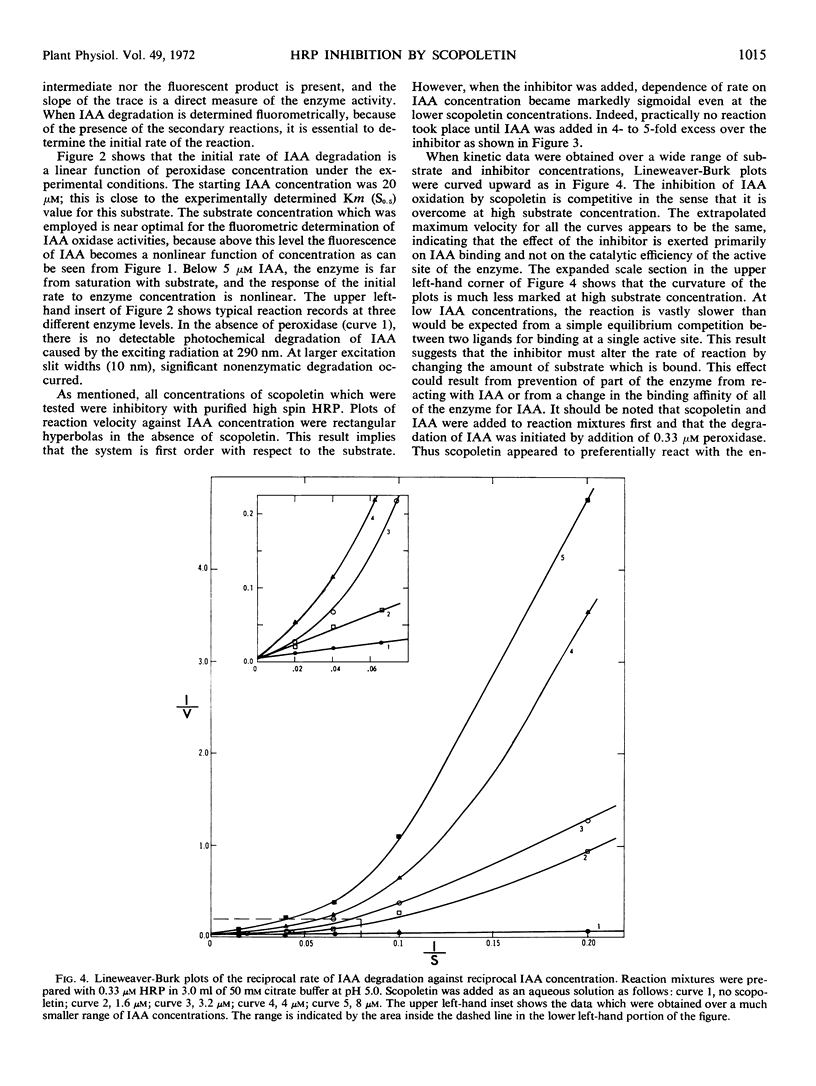
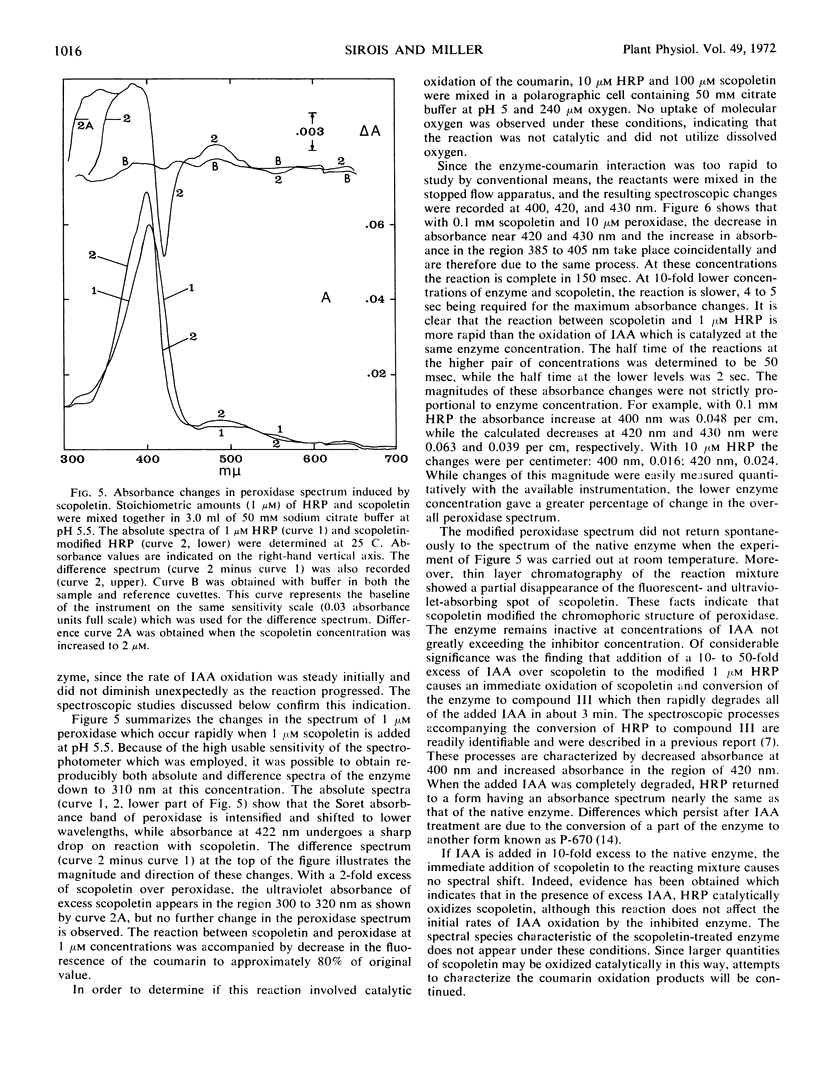
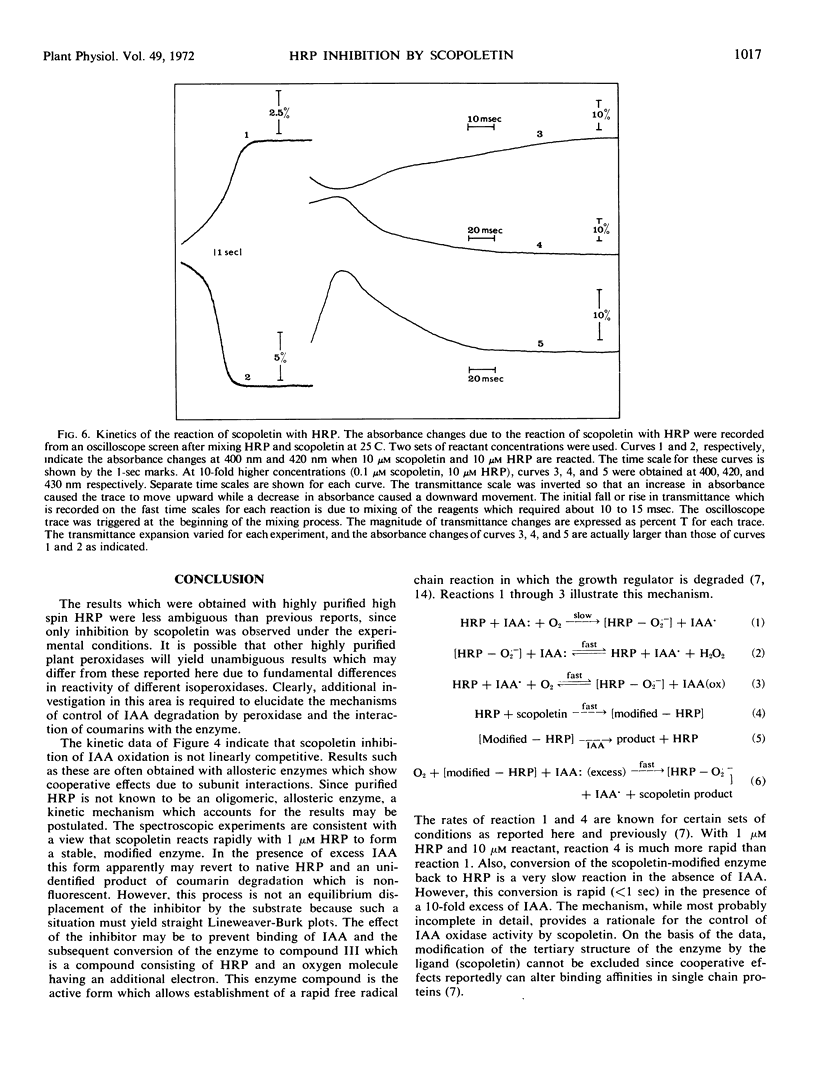
The naturally occurring coumarin, scopoletin, has been found to modify horseradish peroxidase rapidly to give a stable, spectroscopically distinguishable form of the enzyme. Peroxidase treated with scopoletin is less active in reactions with molecular oxygen and indole-3-acetic acid. Kinetic data for the degradation of this growth regulator were obtained with a continuously monitored fluorometric procedure. Lineweaver-Burk plots of the reciprocal rate of degradation against the reciprocal substrate concentration were markedly curved in the presence of the inhibitor, scopoletin. Excess indole-3-acetate restored the scopoletin-treated enzyme to a reactive state. In the presence of molecular oxygen, concentrations of indole-3-acetic acid which were at least 10-fold greater than the inhibitor concentration led to the rapid oxidation of the coumarin and converted peroxidase to compound III as expected from previous studies. This form of the enzyme is the catalytically active species in the oxidative degradation of the growth regulator. The kinetically preferential reaction of scopoletin or related coumarins with peroxidase and the suppression of indole-3-acetic acid degradation may provide a possible control mechanism over the oxidative degradation of indole-3-acetate by this plant enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. Effect of scopoletin on indolacetic acid metabolism. Nature. 1952 Jul 12;170(4315):83–84. doi: 10.1038/170083a0. [DOI] [PubMed] [Google Scholar]
- Fox L. R., Purves W. K., Nakada H. I. The role of horseradish peroxidase in indole-3-acetic acid oxidation. Biochemistry. 1965 Dec;4(12):2754–2763. doi: 10.1021/bi00888a028. [DOI] [PubMed] [Google Scholar]
- HINMAN R. L., BAUMAN C., LANG J. The conversion of indole-3-acetic acid to 3-methyleneoxindole in the presence of peroxidase. Biochem Biophys Res Commun. 1961 Jul 26;5:250–254. doi: 10.1016/0006-291x(61)90156-5. [DOI] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Wittenberg B. A., Wittenberg J. B. The electronic structure of protoheme proteins. 3. Configuration of the heme and its ligands. J Biol Chem. 1968 Apr 25;243(8):1871–1880. [PubMed] [Google Scholar]
- RAY P. M. Destruction of indoleacetic acid. IV. Kinetics of enzymic oxidation. Arch Biochem Biophys. 1962 Feb;96:199–209. doi: 10.1016/0003-9861(62)90399-5. [DOI] [PubMed] [Google Scholar]
- Ricard J., Nari J. The formation and reactivity of peroxidase compound 3. Biochim Biophys Acta. 1967 Mar 15;132(2):321–329. doi: 10.1016/0005-2744(67)90151-9. [DOI] [PubMed] [Google Scholar]
- Schafer P., Wender S. H., Smith E. C. Effect of scopoletin on two anodic isoperoxidases isolated from tobacco tissue culture w-38. Plant Physiol. 1971 Aug;48(2):232–233. doi: 10.1104/pp.48.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]


