Fig. 2.
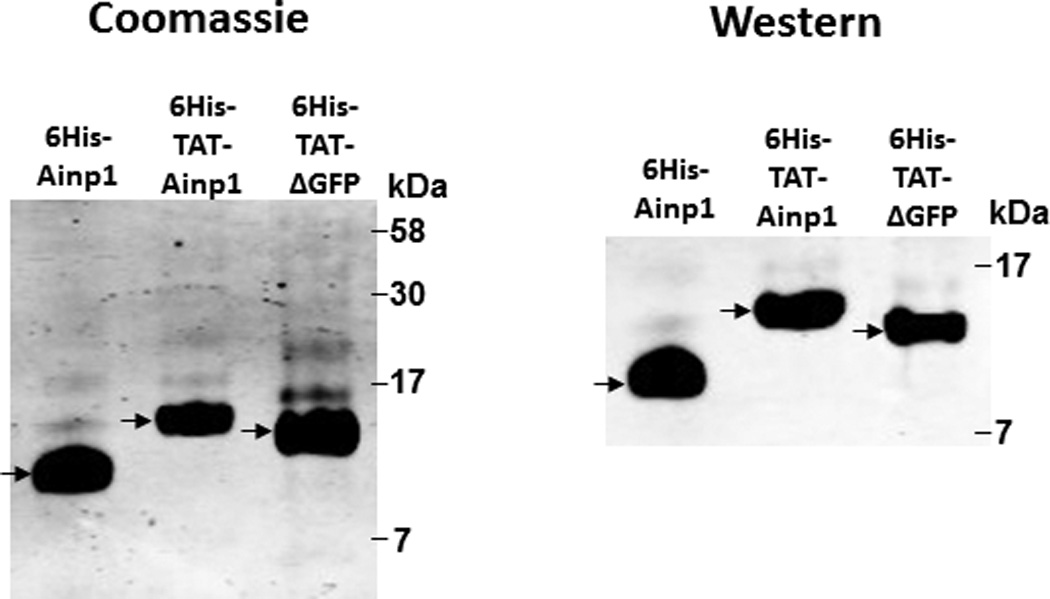
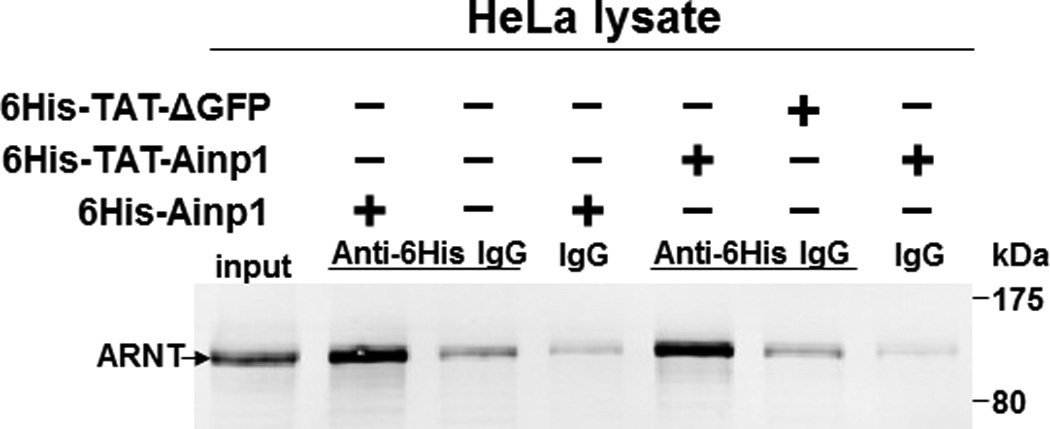
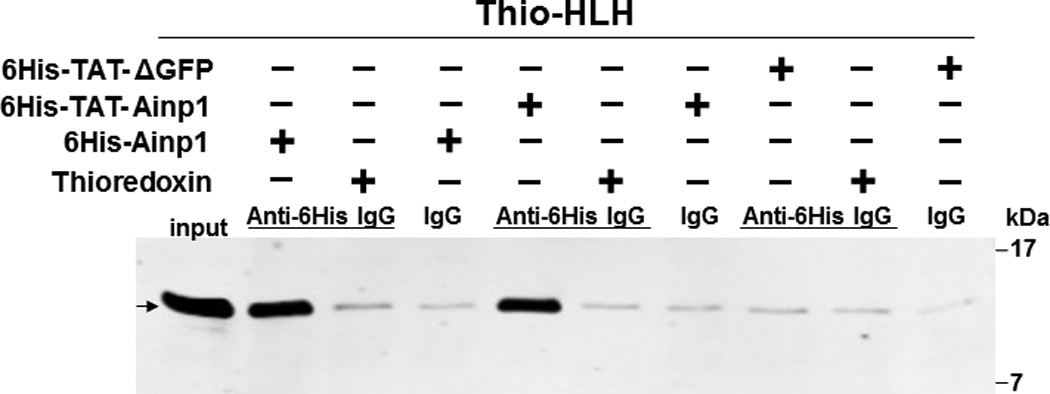
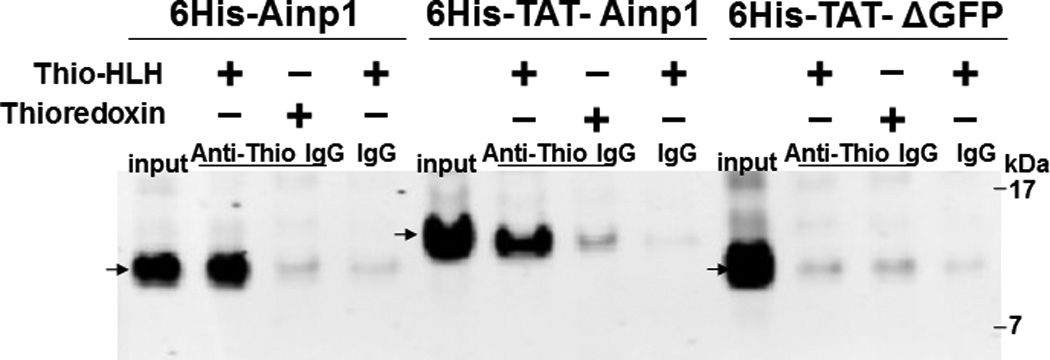
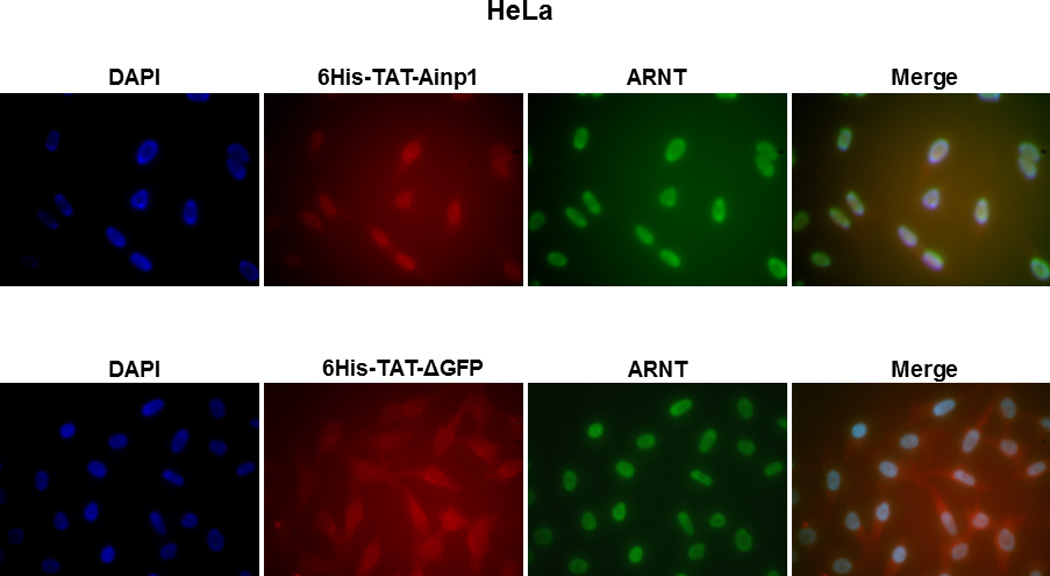
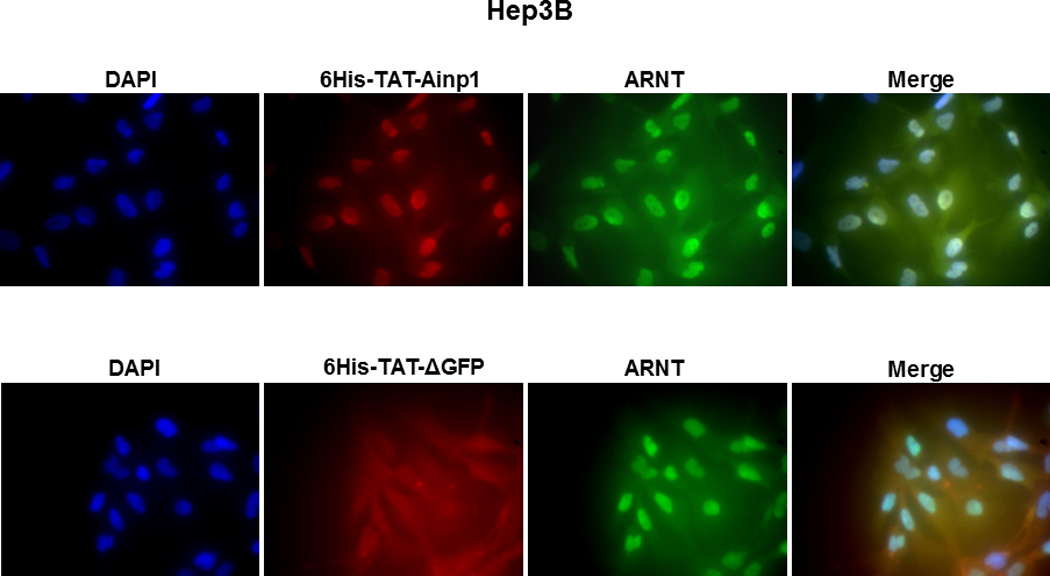
Co-immunoprecipitation studies showing that refolded 6His-TAT-Ainp1 interacted with the HLH domain of ARNT and colocalized with ARNT in the HeLa cell nucleus. Coomassie staining (A, left panel) and western analysis (A, right panel) of soluble 6His-Ainp1 and refolded 6His-TAT-Ainp1 and 6His-TAT-ΔGFP. Each sample was 10 µg for Coomassie and 2 µg for western. 16% tricine gel was used for analysis [39]. Arrows indicate the band of interest. Anti-6His mouse IgG was used to co-immunoprecipitate ARNT from HeLa cell lysate (B) or thioredoxin fusion of ARNT HLH (Thio-HLH) (C). Soluble 6His-Ainp1 (positive control) and refolded 6His-TAT-Ainp1 were used as baits; refolded 6His-TAT-ΔGFP, thioredoxin and mouse IgG as negative controls. (D) Anti-Thio mouse IgG was used to co-immunoprecipitate soluble 6HisAinp1, refolded 6His-TAT-Ainp1 and refolded 6His-TAT-ΔGFP. Thioredoxin fusion of ARNT HLH (Thio-HLH) was used as bait: thioredoxin and mouse IgG were negative controls. Amount of protein used: +, 40 µg of protein. These experiments (B–D) were repeated twice with similar results. Immunofluorescence microscopy of HeLa (E) and Hep3B (F) cells stained for 6His-TAT-Ainp1 (red), 6His-TAT-ΔGFP (red) and ARNT (green). Before the immunofluorescence staining, the cells were treated with either 6His-TAT-Ainp1 (2 µM) or 6His-TAT-ΔGFP (2 µM) for 4 h. DAPI (blue) staining was used to visualize DNA stained nuclei. Merge, image merging analysis. This experiment was repeated once with similar results.
