Abstract
We have recently demonstrated that synthetic CpG oligonucleotides (ODNs), which function as potent immunostimulators, bind to the multi-lectin receptor DEC-205, resulting in their internalization. DEC-205-deficient mice exhibit impaired dendritic-cell and B-cell maturation, impaired cytokine responses and suboptimal cytotoxic T-cell responses. As murine and human DEC-205 are highly conserved, CpG ODNs destined to clinical applications should be designed to maximize DEC-205 binding.
Keywords: dendritic cells, CpG, TLR9, vaccines, adjuvants
Dendritic cells (DCs) detect pathogens and their products using a gamut of receptors,1 including Toll-like receptor 9 (TLR9), which recognizes non-methylated cytosine-guanosine (CpG) motifs found in bacterial DNA.1,2 Synthetic CpG oligonucleotide (ODN) TLR9 agonists are used as vaccine adjuvants.3 Although different classes of CpG ODNs have been developed, clinical trials predominantly utilize class B ODNs (B-ODNs),3 which are single stranded, fully phosphorothioated and activate B cells, plasmacytoid DC (pDCs) and, in the mouse, conventional DCs (cDCs). The phosphorothioate backbone confers resistance to nuclease degradation and facilitates uptake.4,5 The CpG ODN-mediated activation of DCs is known to require TLR9 but it has long remained unclear how extracellular CpG ODNs gain access to the intracellular compartments that house TLR9. We have recently shown that DEC-205 (CD205), a 205 kDa cell-surface molecule bearing 10 C-type lectin-like domains, is a key receptor involved in the uptake of B-ODNs (Fig. 1).6 DEC-205 is expressed in both mice and humans, by various cell types including DCs, B cells and T cells.
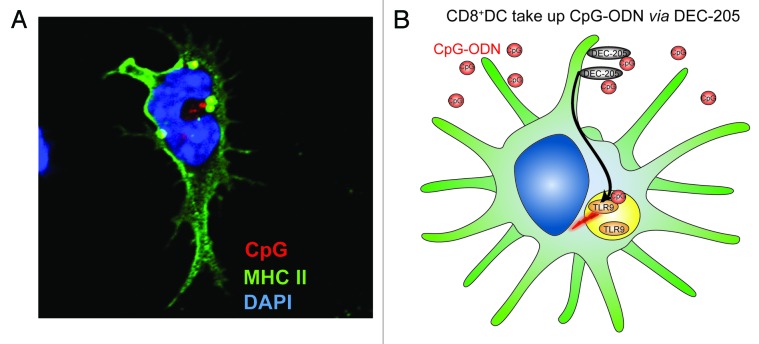
Figure 1. DEC-205 facilitates the uptake of CpG oligodeoxynucleotides. (A) A CD8+ DC bearing intracellular CpG oligodeoxynucleotides (ODN) upon DEC-205-mediated internalization. Nuclei appear in blue, MHC Class II molecules in green and a fluorescent CpG ODN in red. Yellow zones constitute areas in which CpG ODN has co-localized with MHC Class II molecules. (B) Role of DEC-205 in the capture and internalization of CpG ODNs by CD8+ DCs.
Utilizing three distinct assays, we have demonstrated that DEC-205 directly interacts with CpG ODNs.6 By flow cytometry, we have shown that cells that normally do not express DEC-205 become able to specifically bind CpG ODNs upon DEC-205 plasmid-driven expression. In line with this notion, soluble DEC-205, but not other C-type lectins, was shown to bind CpG ODNs by ELISA and surface plasmon resonance. Interestingly, whereas CpG motifs are required for the activation of TLR9 by CpG ODNs,7 the same does not apply to DEC-205 binding. Arguably, the most important finding that we have reported is that human DEC-205 also binds CpG ODNs, but—interestingly—does so in a selective fashion.6 Thus, while mouse DEC-205 bound equally well various B-ODNs, human DEC-205 preferentially bound CpG ODN 2006, which is the ODNs currently used in clinical trials. This observation suggests DEC-205 binding contributes to the efficacy of CpG ODN 2006 in vivo.
To assess the biological significance of the interaction between DEC-205 and CpG ODNs, we compared the response of DEC-205-deficient and wild-type mice to these agents. We observed that CD8+ DCs, which express the highest levels of DEC-205, actively use this receptor to capture and internalize CpG ODNs in vivo. Normally, when DCs detect danger signals, such as CpG ODNs, they mature while upregulating molecules that promote antigen presentation. This was not the case for CD8+ DCs lacking DEC-205, which did not respond to CpG ODNs by upregulating the antigen-presentation machinery (i.e., CD40, CD80, MHC class II molecules) to the same extent than wild type CD8+ DCs. These observations indicate that DEC-205 is required for optimal DC responses to CpG ODNs. B cells also present antigens to T cells, express DEC-205 and are activated by CpG ODNs. Importantly, just like DCs, B cells turned out to require DEC-205 to optimally respond to CpG ODNs and upregulate the factors that are required for T-cell activation (i.e., CD40, CD86 and MHC class II molecules). While suboptimal antigen presentation can affect the priming of adaptive immune responses, DCs are also involved in innate immune responses. Indeed CpG ODNs induce the rapid production of pro-inflammatory cytokines. We have demonstrated that DEC-205 is required for the optimal induction of various cytokines and chemokines, including interleukin(IL)-1α, IL-6, tumor necrosis factor α (TNFα), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)-1α and MIP-1β, in response to CpG ODNs. Most strikingly though was the poor production of IL-12, a cytokine produced in large amounts by CD8+ DCs.6
Given that CD8+ DCs play a key role in activating naïve CD8+ T cells and IL-12 promotes the acquisition of cytotoxic functions, and that DEC-205-deficient mice exhibited impaired CD8+ DC maturation and an impaired ability to produce IL-12 in response to CpG ODNs, we wondered whether DEC-205 is required for the induction of cytotoxic T lymphocytes (CTLs) in a model that depends on CpG ODNs for adjuvancy. To test this hypothesis, we immunized mice with CpG ODN together with a monoclonal antibody specific for Clec12A coupled to ovalbumin. Since Clec12A is expressed on DCs, this immunization regimen delivers ovalbumin to DCs, yet it requires the adjuvant effect of CpG ODNs for the successful priming of CTLs.8 With this approach, we have shown that the induction of robust CTL responses that depend on the adjuvant effect of CpG ODNs also requires DEC-205.6
Thus, DEC-205 is required for the induction of strong pro-inflammatory responses to CpG ODNs in mice. As this receptor is well conserved in humans, DEC-205 may also play an important role in promoting optimal responses to CpG ODNs in clinical settings. Since the capacity of human DEC-205 to bind CpG ODNs heavily depends on their DNA sequence, it will be important to identify the motifs that most efficiently bind DEC-205 so as to facilitate optimal uptake. In man, the main targets of CpG ODNs are B cells and pDCs, both of which express TLR99 and DEC-205.10 Thus, identifying the ideal sequence for human DEC-205 binding and coupling this to the ideal sequence for TLR9 activation should allow for the design of CpG ODNs with maximal stimulatory capacities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23128
References
- 1.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 2.Wagner H. The sweetness of the DNA backbone drives Toll-like receptor 9. Curr Opin Immunol. 2008;20:396–400. doi: 10.1016/j.coi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J Immunol. 2000;165:4165–73. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- 5.Roberts TL, Dunn JA, Sweet MJ, Hume DA, Stacey KJ. The immunostimulatory activity of phosphorothioate CpG oligonucleotides is affected by distal sequence changes. Mol Immunol. 2011;48:1027–34. doi: 10.1016/j.molimm.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Lahoud MH, Ahmet F, Zhang JG, Meuter S, Policheni AN, Kitsoulis S, et al. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci U S A. 2012;109:16270–5. doi: 10.1073/pnas.1208796109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas T, Metzger J, Schmitz F, Heit A, Müller T, Latz E, et al. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–23. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. 2011;187:842–50. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 9.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–69. doi: 10.1093/intimm/dxl022. [DOI] [PubMed] [Google Scholar]