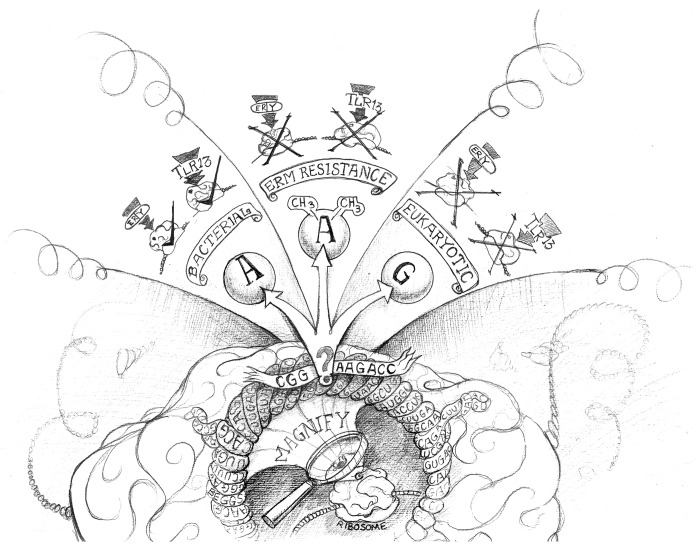
Figure 1. Interaction of erythromycin and Toll-like receptor 13 with the bacterial but not the eukaryotic ribosome. The magnification of the ribosome at the central peptidyl exit tunnel highlights the highly conserved sequence “CGGAAAGACC” (highlighted as banner) as macrolides, lincosamides and streptogramin (MLS) antibiotic- as well as TLR13-binding site. The question mark depicts the adenosine A2058/2085. If this site is an unmodified adenosine (left panel) it will allow erythromycin (ery) to bind and block protein biosynthesis. At the same time, this sequence is being detected by Toll-like receptor 13 (TLR13) (indicated with a tick “√”). In erm-driven resistance (middle panel) is the A2058/2085 methylated. The methyl groups prevent binding of erythromycin, causing both antibiotic resistance and preventing detection via TLR13 (indicated with an “X”). The right panel illustrates substitution of A2058/2085 by guanosine as is operative in eukaryotic 28S rRNAs and bacterial 23S rRNAs of some MLS antibiotic-resistant bacteria, which abrogates erythromycin binding as well as bacteria detection via TLR13 (indicated with an “X”). Figure designed by Derek Beggs (Bavarian-Nordic, Martinsried, Germany).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.