Figure 8. Model for PKCε-initiated metabolic changes linked to cardioprotection.
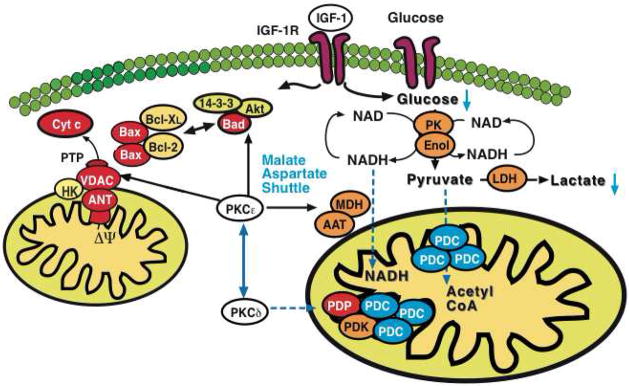
PKCε activation resulted in differential expression of pyruvate kinase (PK), enolase (Enol) and lactate dehydrogenase (LDH) with a corresponding reduction of glucose and lactate in murine hearts. Notably, a pronounced effect was also observed on cytosolic malate dehydrogenase (MDH) and aspartate aminotransferase (AAT), the two enzymatic components of the malate-aspartate shuttle. The latter transfers electrons from the cytoplasm to mitochondria and is important in allowing maximum release of the free energy in glycolysis under aerobic conditions. In contrast, inhibition of PKCε stimulated loop phosphorylation and translocation of PKCδ to cardiac mitochondria, where it targets the pyruvate dehydrogenase complex (PDC), the enzyme responsible for converting the glycolytic endproduct pyruvate to acetyl-CoA. The pyruvate dehydrogenase complex is located at the inner mitochondrial membrane and inhibited when phosphorylated by pyruvate dehydrogenase kinase (PDK) and activated upon dephosphorylation by pyruvate dehydrogenase phosphatase (PDP). Thus, PKCε and PKCδ activities influence key enzymatic reactions bridging aerobic and anaerobic glucose metabolism.
