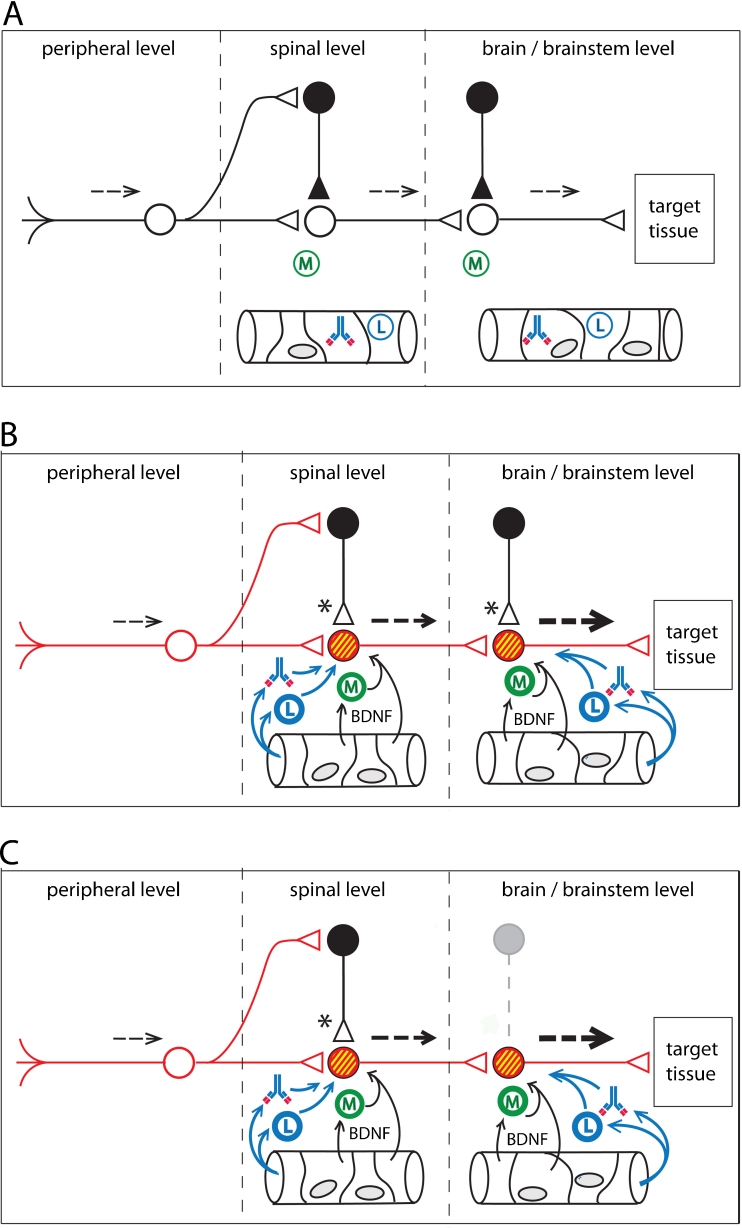
Fig. 2.
A conceptual model for functional pathophysiologies arising from neuroinflammatory tracks. Neuroinflammation leads to a loss of sensory gating, as well as excessive gain, in sensorimotor pathways. a. Normal sensory processing (dotted lines). GABAergic interneurons provide inhibitory tone at both first order and second order synapses. Non-activated microglia (M) lie in the CNS parenchyma. Leukocytes (L) and autoantibodies are present in the CNS vasculature. b. Peripheral injury initiates anterograde central sensitization. Microglial activation takes place in response to cytokines released at the terminals of injured primary afferents (first order synapse). Breakdown of the blood–brain barrier (or the blood-spinal cord barrier) allows activated leukocytes and autoantibodies to extravasate into the parenchyma of the CNS, leading to autoimmune mediated neuroinflammation. Release of Brain-Derived Neurotrophic Factor (BDNF) from plasma, endothelial cells, and activated microglia alter chloride homeostasis in post-synaptic neurons. Accumulation of intracellular chloride (yellow) results in synaptic conversion, changing GABAergic and glycinergic synapses from inhibitory tone to excitatory tone (Huberfeld et al. 2007; Price et al. 2009; Cooper and Przebinda 2011). Sensory gating is lost at the first order synapse. Graded sensory transmission is lost within the neural circuit. Upregulation (red) of excitatory transducers (e.g. glutamate receptors) results an increase in excitatory tone. Functional changes in the circuit result in a collapse of feedforward inhibition and filtering at the first-order synapse (pain gate in the nociceptive circuit) (Cooper and Przebinda 2011). Neuroimmune activation spreads to the second order synapse through the release of the cytokine CCL21 from the terminals of first order neurons (Saab and Hains 2009). Small sensory stimuli provoke large (thick dotted arrows) volleys in second order neurons. c. Central injury to the CNS can result in retrograde central sensitization via neuroimmune activation. Loss of inhibitory interneurons (grey outlines), either by autoimmune attack (e.g. Stiff-Person Syndrome) (Sandbrink et al. 2003; Rokocevic and Floeter 2012), or by metabolic crisis (e.g. hypoglycemia, hypoxia, or excitotoxicity), permanently reduces inhibitory tone in the sensorimotor circuit. These conditions, and/or the conditions illustrated in panel B, may contribute to post-traumatic dystonias, non-dermatomal pain distributions, autonomic, and/or somatovisceral disorders, in patients with autoimmune-mediated neuroinflammation, even after the initial neuroinflammatory event subsides