Abstract
Graphene has attracted great interest because of unique properties such as high sensitivity, high mobility, and biocompatibility. It is also known as a superior candidate for pH sensing. Graphene-based ion-sensitive field-effect transistor (ISFET) is currently getting much attention as a novel material with organic nature and ionic liquid gate that is intrinsically sensitive to pH changes. pH is an important factor in enzyme stabilities which can affect the enzymatic reaction and broaden the number of enzyme applications. More accurate and consistent results of enzymes must be optimized to realize their full potential as catalysts accordingly. In this paper, a monolayer graphene-based ISFET pH sensor is studied by simulating its electrical measurement of buffer solutions for different pH values. Electrical detection model of each pH value is suggested by conductance modelling of monolayer graphene. Hydrogen ion (H+) concentration as a function of carrier concentration is proposed, and the control parameter (Ƥ) is defined based on the electro-active ions absorbed by the surface of the graphene with different pH values. Finally, the proposed new analytical model is compared with experimental data and shows good overall agreement.
Keywords: Graphene, Ion-sensitive field-effect transistor (ISFET), pH sensor, Conductance
Background
Graphene has two sp2-bonded carbon atoms, which make its structure apparently look like a honeycomb crystal as seen in Figure 1[1-3]. Because of its unique properties, graphene has attracted huge interest mainly in the electrical, physical, chemical, and even biological fields [4,5].
Figure 1.
Monolayer graphene atom arrangement with only one atom thickness.
Nowadays, ion-sensitive field-effect transistors (ISFETs) have caught much attention due to their advantages such as small size and the possibilities for mass production [6,7]. Their short and consistent response times are very favorable to the electronics industry [8,9]. ISFETs introduce new features such as the integration of data processing and compensation circuits in the similar circuit for this type of sensors [10-12]. By altering the gate material, depositing layers of selective membrane or a bio-recognition element onto the gate, variance of selectivity can be achieved [13,14]. After the process of depositing, the sensors are now called chemically sensitive FETs [15,16]. Initially, heterogeneous membranes of silver halides and membranes based on polyvinyl chloride (PVC) have been used for ISFET [17,18]. Due to poor adherence between PVC base membrane and ISFET surface and inconsistent results, scientists explore for a new type of membrane [18,19]. That is where photocured polymers, which are compatible with the proposed photolithography techniques, come in [19,20]. They have the properties of a higher adherence string of the salinized ISFET gate's surface [21]. In order to expand ion-selective membranes, numerous polymers such as polysiloxanes, polyurethanes, and different methacrylate-derived polymers have been reported to be good candidates [22,23]. These new polymers show promising results regarding consistency and longer stability compared to PVC membranes [24]. In addition, almost all effective ion-based ISFETs were developed for clinical analyses and environmental applications [24]. Recently, microelectronic advances have been exploited and applied to improve ISFET fabrication methods [25,26]. Because of the electrolyte's ionic properties, electrical parts of ISFETs cannot have contact with liquid and only the gate area is open [27]. Due to its organic nature, the gate material for ISFETs is intrinsically sensitive to pH changes [28,29]. On the other hand, all enzymes are sensitive to pH changes, but extremely high or low pH values can make these enzymes lose their sensitivity [30,31]. pH is also a main factor in enzyme stabilities [32]. Each enzyme includes a suitable or optimal pH stability range [30,32]. Apart from temperature and pH, ionic strength can also affect the enzymatic reaction [33]. For more accurate and consistent results, each of these physical and chemical parameters must be considered and optimized accordingly [34]. ISFETs can be based on many materials as their detectors such as membranes and graphene [35]. Because of the physical and electrical properties of graphene, it can be applied as a sensing material in the structure of FETs [35]. On the other hand, there are no information on the development and modelling of ion-sensitive FETs, and their potential as ISFET has not been totally studied yet. The reaction between solution with different pH values and the surface of graphene has a notable effect on the conductivity of graphene [36]. This means that the detection mechanism of adsorbing the hydrogen ions from solution to carbon-based materials can be clarified as shown in Figure 2. In other words, based on the electron transfer between ion solutions and graphene surface, an analytical model of the reaction between buffer solution of different pH and graphene is presented.
Figure 2.
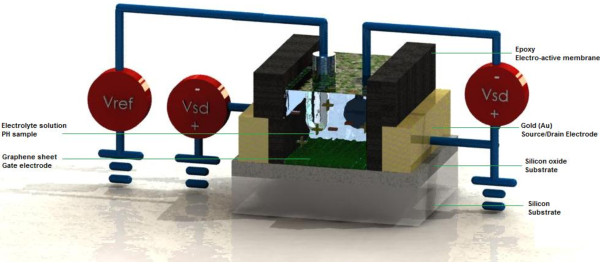
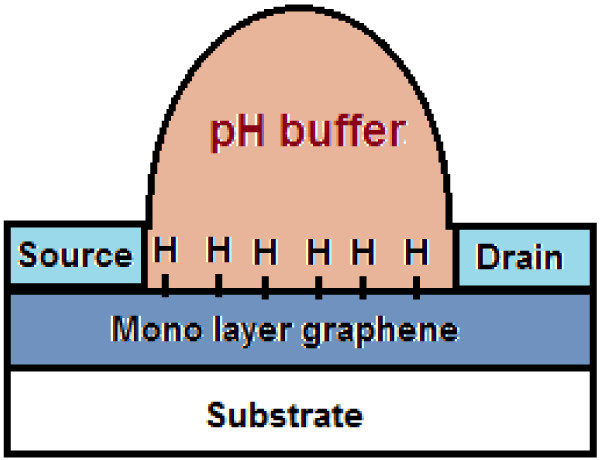
Schematic of the proposed structure and the electrical circuit of graphene based-ISFET for pH detection.
Figure 2 illustrates the detection mechanism of solution with different pH using an ISFET device. Monolayer graphene on silicon oxide and silicon substrate with a deposited epoxy layer (Epotek 302–3 M, Epoxy Technology, Billerica, MA, USA) as an ISFET membrane is proposed. In this paper, pH of solution as a gate voltage is replicated due to the carrier injected to channel from it, and also pH as a sensing parameter (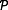 ) is suggested. Finally, the presented model is compared with experimental data for purposes of validation.
) is suggested. Finally, the presented model is compared with experimental data for purposes of validation.
Proposed model
The graphene nanoribbon channel is supposed to be completely ballistic for one-dimensional monolayer ISFETs for pH sensing since high carrier mobility has been reported from experiments on graphene [37]. A district of minimum conductance versus gate voltage as a basic constant relative to the electron charge in bulk graphite (q) and Planck's constant (h) is defined by G0 = 2q2/h[38]. So, the electron transportation of the graphene channel in ISFET can be obtained by the Boltzmann transport formula [38,39]:
| (1) |
where E is the energy band distribution, T(E) is the average probability of electron transmission in the channel between source and drain which is equal to 1 (T(E) = 1) [38] because the ballistic channel is assumed for the ISFET device, f is the Fermi-Dirac distribution function, and M(E) is the number of sub-bands in the ISFET channel as a summation parameter over k point which is defined as
| (2) |
where l is the ISFET channel length, t = 2.7 eV which is the tight-binding energy for the nearest neighbor C-C atoms, and β is the quantized wave vector which can be written as
| (3) |
where N is the number of dimer lines, Pi is the modulation index, and ac−c = 1.42 Å is the distance between adjacent carbon atoms in the plan. The conductance of the nanoscale material is strongly dependent on both quantizing parameters, which depend on the number of sub-bands, and interface resistance, which is independent of the length [40]. Also, the Fermi-Dirac distribution function is inserted instead of the number of sub-bands in the ISFET channel. So, it is modified as
| (4) |
In order to simplify the conductance equation, we assumed x = (E − Eg / kBT) and η = (EF − Eg) / kBT as normalized Fermi energy. Consequently, the supposed conductance model of the graphene-based ISFET channel can be written as
| (5) |
This equation can be numerically solved for different gate voltages. Thus, the proposed conductance model of the performance of the graphene-based ISFET in the nanostructured region by the conductance-voltage characteristic is evaluated in Figure 3.
Figure 3.
A bipolar transfer curve of the conductance model of graphene-based ISFET.
By applying gate voltage between 0.2 and 0.7 V, a bipolar characteristic of FET device is monitored since the Fermi energy can be controlled by gate voltage. Based on this characteristic, it is notable that the graphene can be continuously dropped from the p-doped to the n-doped region by the controllable gate voltage. The minimum conductance is observed at the transition point between electron and hole doping. This conjunction point is called the charge-neutrality point (CNP) [41]. The conductance of the ISFET channel not only is dependent on the graphene structure and operation voltage on the source-drain channel, but also depends on the electrolyte environment and ion concentration in solution [42,43]. It has been demonstrated that different pH values can affect the ISFET conductance [42].
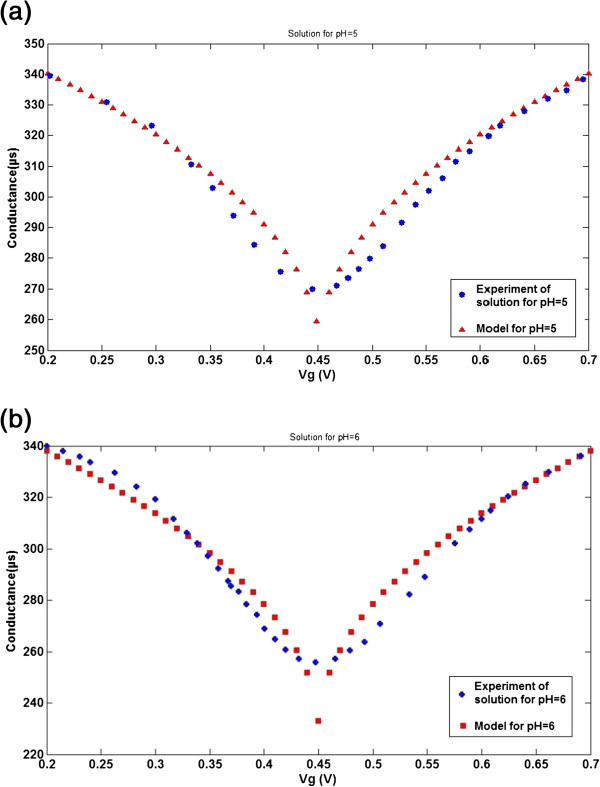
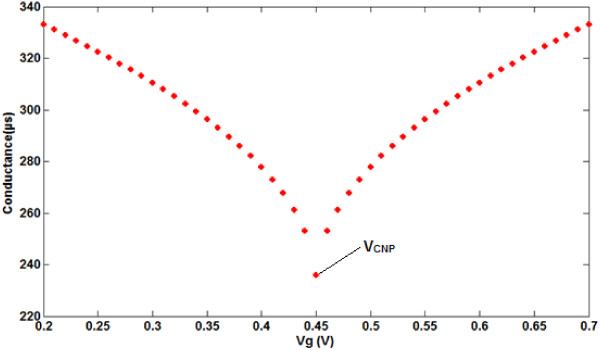
Before the hydrogen ion concentration was changed in the solution, a natural solution (pure water) with a buffer (pH = 7) was added in the electro-active membrane to measure the dependence of conductance versus gate voltage. There is a favorable agreement between the proposed model for pH sensing based on graphene and experimental data for non-ionic solution (pH = 7) which are extracted from [42], as can be seen in Figure 4.
Figure 4.
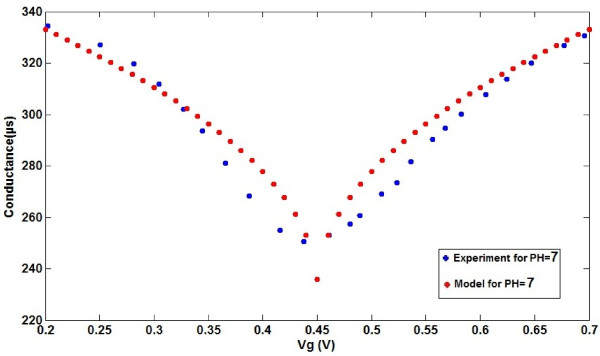
Electrical source-drain conductance versus gate voltage of graphene-based ISFET for both model and experimental data.
The conductivity of the graphene-based ISFET device is influenced by the number of carriers changing in the channel. A graphene-based ISFET with high sensitivity is applied to detect the different pH values based on conductance altering [42]. As can be seen in Figure 5, the conductance of the channel changes due to the binding of hydrogen ions in the solution to the surface of the ISFET channel. When the pH value of the solution rises from 5 to 10, less hydrogen ions will be adsorbed and the sensor will be capable of attracting less ions, leading to changes in the conductance of the graphene-based ISFET, as shown in Figure 6.
Figure 5.
Schematic of hydrogen ion adsorption processes by surface area of single-layer graphene.
Figure 6.
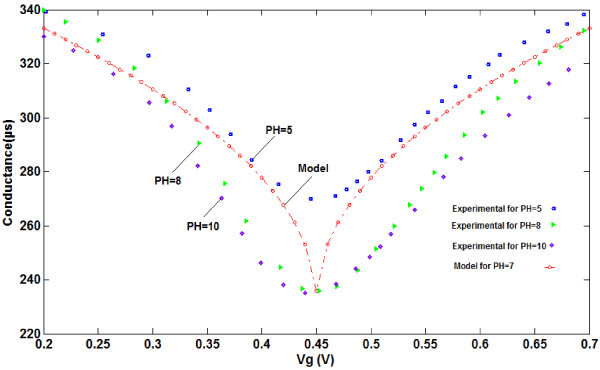
Comparison between graphene conductance model and extracted experimental data[42]for different pH values.
Dependent upon the source-drain conductance of the ISFET device, we can write
| (6) |
The focus of this paper is to present a new model for ISFET to measure pH changes; in other words, the conductance of the ISFET device as a function of different pH values is simulated and the pH factor (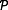 ) is suggested. Subsequently, for more understanding of the role of hydrogen ion concentration, FET modelling is employed to obtain an equation between the conductance and pH of a solution, where the suggested structure of ISFET is shown in Figure 2 with source and drain as contacts. Ultimately, different pH values can be modelled by the pH of a solution (see the following equation). This means that Gwith pH can be shown as a function of pH values:
) is suggested. Subsequently, for more understanding of the role of hydrogen ion concentration, FET modelling is employed to obtain an equation between the conductance and pH of a solution, where the suggested structure of ISFET is shown in Figure 2 with source and drain as contacts. Ultimately, different pH values can be modelled by the pH of a solution (see the following equation). This means that Gwith pH can be shown as a function of pH values:
| (7) |
where the pH sensing factor (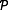 ) is assumed and PH is the pH value. In the non-saturation region, the ISFET conductance model is shown as a function of gate voltage and the ideal conductance-voltage relation to the graphene channel of the ISFET device from Equations 5 and 7:
) is assumed and PH is the pH value. In the non-saturation region, the ISFET conductance model is shown as a function of gate voltage and the ideal conductance-voltage relation to the graphene channel of the ISFET device from Equations 5 and 7:
| (8) |
So, the G-Vg characteristics of both the model and experimental data of graphene-based ISFET for changing the pH level in solution from 6 to 7 are plotted in Figure 7.
Figure 7.
G-Vg characteristics of proposed conductance model with experimental data[42]. For solutions with (a) pH = 5 and (b) pH = 6.
By comparing the suggested ISFET modelling based on the proposed parameter model with experimental data in Figure 7, similar trends can be considered. In order to show all figures without overlapping, each pH value has been plotted respectively in Figure
7
a,b. In addition, a detailed comparison between observed new models per pH is illustrated in Figure 7, which demonstrates acceptable agreement with experimental data. In the suggested model, different pH values is demonstrated in the form of 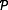 parameter which is in agreement with the reported data, as shown in Table 1.
parameter which is in agreement with the reported data, as shown in Table 1.
Table 1.
Different pH values with Ƥ parameter
| Ƥ parameter values | pH values |
|---|---|
| 0.039105 |
5 |
| 0.035142 |
6 |
| 0.034918 |
7 |
| 0.034662 |
8 |
| 0.034437 |
9 |
| 0.034209 | 10 |
Therefore, based on the iteration method in Table 1, the electro-active ions absorbed by the surface of the ISFET channel as a pH sensing factor (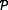 ) can be suggested by the following equations:
) can be suggested by the following equations:
| (9) |
| (10) |
According to the saturation region of the proposed conductance model belonging to the ISFET device, Equation 11 is acceptable for both the saturation behavior and experimental data from [42]:
| (11) |
From extracted data, α and β parameters are calculated, where α = 2.7318 and β = 4.5044. Consequently, based on the proposed model of the ISFET device, the conductance versus gate voltage is modified as
| (12) |
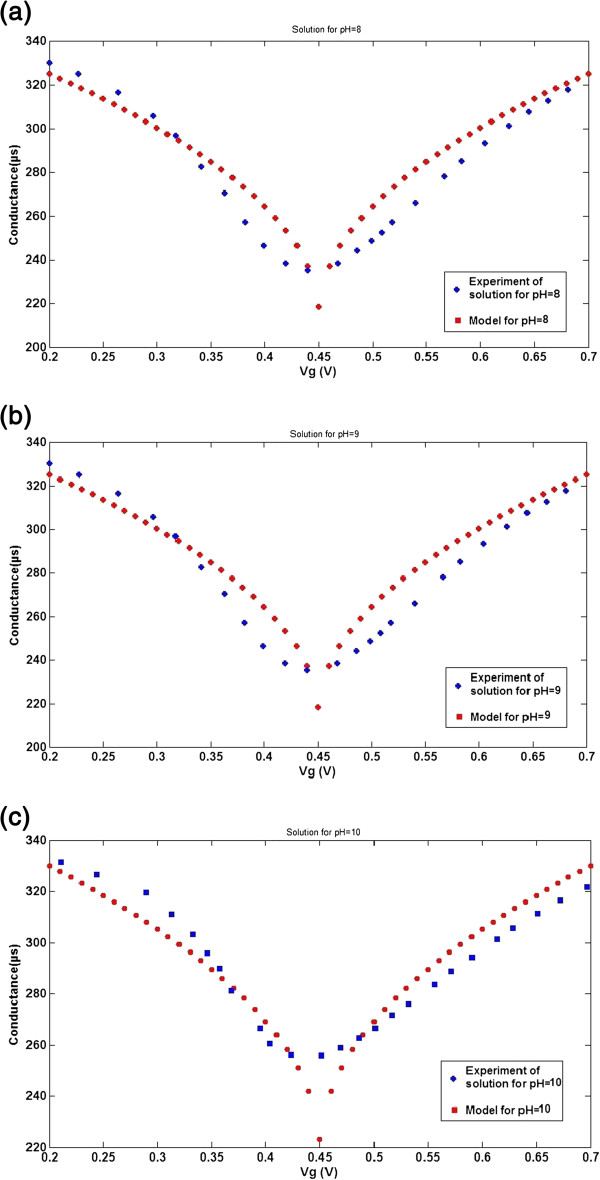
As can be seen in Figure 8, the theoretical G-Vg characteristics of graphene-based ISFET for pH changes from 8 to 10 are plotted.
Figure 8.
G-Vg characteristics of the proposed conductance model with experimental data. For solutions with (a) pH = 8, (b) pH = 9, and (c) pH = 10.
It is evident that the G-Vg characteristic curve can be controlled by the pH factor (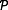 ) and also the proposed model of ISFET conductance closely matches with experimental data. In both reported data and theoretical data, the decline of ISFET conductance is noticeable when the pH level increases. Also, the conductance curve is almost symmetric near VCNP, while at a large carrier concentration of about 350 to 400 μS, a saturation behavior is depicted. Comparing both experimental data and theoretical data depicted in Figure 5 reveals that when the concentration of hydrogen ions changes from pH = 7 to pH = 8, ISFET conductance decreases about 5 μS. Also, as shown in Figure 8a,b,c, each graph shows a particular value of pH. For example, when the pH value is 8, it is notable that the model is closer to the blue line (experimental data), and also in the different pH values, we can compare other ion concentrations as well. An innovative analysis of matching models using the different values in experimental data is presented in this work to verify that the conductivity of the graphene-based ISFET is moved down vertically at higher pH values. The ion-sensitive FET structure was used with monolayer graphene prepared by CVD and grown in large size on pieces of p-doped Si covered with a 300-nm substrate to measure pH changes [42]. In this study, one can claim that pH changes in the electro-active membrane will significantly and vertically shift the value of conductance in graphene (Gwith pH) that occurred due to ion adsorption on the surface area of the monolayer graphene sheet of the ISFET channel. Also, it is notable that the temperature remains constant (about 25°C in solution) in the suggested model as the temperature can have an effect on the behavior of the sensing parameter as well.
) and also the proposed model of ISFET conductance closely matches with experimental data. In both reported data and theoretical data, the decline of ISFET conductance is noticeable when the pH level increases. Also, the conductance curve is almost symmetric near VCNP, while at a large carrier concentration of about 350 to 400 μS, a saturation behavior is depicted. Comparing both experimental data and theoretical data depicted in Figure 5 reveals that when the concentration of hydrogen ions changes from pH = 7 to pH = 8, ISFET conductance decreases about 5 μS. Also, as shown in Figure 8a,b,c, each graph shows a particular value of pH. For example, when the pH value is 8, it is notable that the model is closer to the blue line (experimental data), and also in the different pH values, we can compare other ion concentrations as well. An innovative analysis of matching models using the different values in experimental data is presented in this work to verify that the conductivity of the graphene-based ISFET is moved down vertically at higher pH values. The ion-sensitive FET structure was used with monolayer graphene prepared by CVD and grown in large size on pieces of p-doped Si covered with a 300-nm substrate to measure pH changes [42]. In this study, one can claim that pH changes in the electro-active membrane will significantly and vertically shift the value of conductance in graphene (Gwith pH) that occurred due to ion adsorption on the surface area of the monolayer graphene sheet of the ISFET channel. Also, it is notable that the temperature remains constant (about 25°C in solution) in the suggested model as the temperature can have an effect on the behavior of the sensing parameter as well.
Conclusions
Graphene with sp2-bonded carbon atoms has considerable potential on bio-sensing materials and electrochemical applications. The emerging potentials of nanostructured graphene-based ISFETs with high sensitivity and ability to readily detect have been applied to electrochemical catalysis through pH sensing. The conductance of an ISFET device with different pH values can be displayed by the ion concentration of the solution. In this research, the conductance of graphene is assumed as a function of pH levels (Gwith pH ≈ pH), which shows the pH factor. Measurements show decreasing conductivity when the pH value of the electrolyte is increased. Especially in VCNP, the changed conductance values are clearly depicted. The suggested model verifies the reported experimental data as well. In other words, based on the good agreement between the presented analytical model and experimental data, 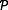 can be seen as a pH factor to predict graphene behavior in graphene-based ISFETs.
can be seen as a pH factor to predict graphene behavior in graphene-based ISFETs.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MJK wrote the manuscript and contributed to the analytical modelling of the presented FET via MATLAB software. Dr. FKCh and Dr. MTA revised the manuscript and coordinated between all the contributors. HKFA, MR, and AH organized the final version of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Mohammad Javad Kiani, Email: kianiph@yahoo.com.
Mohammad Taghi Ahmadi, Email: taghi@fke.utm.my.
Hediyeh Karimi Feiz Abadi, Email: hediyeh.karimi@gmail.com.
Meisam Rahmani, Email: meisamrahmani313@gmail.com.
Amin Hashim, Email: cik.amin@gmail.com.
Fauzan Khairi Che harun, Email: fauzan@fke.utm.my.
Acknowledgments
The authors would like to acknowledge the financial support from the Research University grant of the Ministry of Higher Education (MOHE), Malaysia, under Project Q.J130000.7123.02H24. Also, thanks to the Research Management Center (RMC) of Universiti Teknologi Malaysia (UTM) for providing excellent research environment in which to complete this work.
References
- Wen Xu YG, Liwei L, Hua Q, Yanli S. Can graphene make better HgCdTe infrared detectors? Nanoscale Res Lett. 2011;8:250. doi: 10.1186/1556-276X-6-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelo Vecchio SS, Corrado B, Rambach M, Rositza Y, Raineri V, Filippo G. Nanoscale structural characterization of epitaxial graphene grown on off-axis 4H-SiC (0001) Nanoscale Res Lett. 2011;8:269. doi: 10.1186/1556-276X-6-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An XS, Trevor John S, Rakesh W, Christopher L, Washington KM, Morris N, Talapatra SK, Saikat K, Swastik Q. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010;8(11):4295–4301. doi: 10.1021/nl903557p. [DOI] [PubMed] [Google Scholar]
- Myung SS, Aniruddh K, Cheoljin P, Jaesung K, Lee KS, Ki-Bum. Graphene-encapsulated nanoparticle-based biosensor for the selective detection of cancer biomarkers. Adv Mater. 2011;8(19):2221–2225. doi: 10.1002/adma.201100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AD, Viet NA. A new type of optical biosensor from DNA wrapped semiconductor graphene ribbons. J Appl Phys. 2012;8(11):114703. doi: 10.1063/1.4728196. [DOI] [Google Scholar]
- Pham MTH, Kunath S, Kurth C, Köhler E, Howitz S. Backside membrane structures for ISFETs applied in miniature analysis systems. Sensors and Actuators B: Chemical. 1994;8(1–3):333–335. [Google Scholar]
- Gotoh M, Suzuki M, Kubo I, Tamiya E, Karube I. Immuno-FET sensor. J Mol Catal. 1989;8(3):285–292. doi: 10.1016/0304-5102(89)80063-X. [DOI] [Google Scholar]
- Schlesinger R, Bruns M, Becht R, Dosenbach S, Hoffmann W, Ache HJ. ISFETs with sputtered sodium alumino-silicate glass membranes. Fresenius J Anal Chem. 1996;8(7–8):852–856. doi: 10.1007/s0021663540852. [DOI] [PubMed] [Google Scholar]
- Lee D, Cui T. An electric detection of immunoglobulin G in the enzyme-linked immunosorbent assay using an indium oxide nanoparticle ion-sensitive field-effect transistor. J Micromech Microeng. 2012;8(1):015009. doi: 10.1088/0960-1317/22/1/015009. [DOI] [Google Scholar]
- Chen SC, Su Y-K, Tzeng JS. The fabrication and characterisation of ion-sensitive field-effect transistors with a silicon dioxide gate. J Phys D: Appl Phys. 1986;8(10):1951. doi: 10.1088/0022-3727/19/10/020. [DOI] [Google Scholar]
- Shepherd L, Toumazou C. Weak inversion ISFETs for ultra-low power biochemical sensing and real-time analysis. Sensors and Actuators B: Chemical. 2005;8(1):468–473. doi: 10.1016/j.snb.2004.11.006. [DOI] [Google Scholar]
- Chung W-YL, Yeong-Tsair P, Yang DG, Chung-Huang W, Ming-Chia K, Alfred T, Wladyslaw Q. ISFET interface circuit design with temperature compensation. Microelectron J. 2006;8(10):1105–1114. doi: 10.1016/j.mejo.2006.05.001. [DOI] [Google Scholar]
- Kal SB, Bhanu PV. Proceedings of TENCON 2007–2007 IEEE Region 10 Conference: October 30 - November 2; Taipei. Piscataway: IEEE; 2007. Design and modeling of ISFET for pH sensing; pp. 1–4. [Google Scholar]
- Voigt H, Schitthelm F, Lange T, Kullick T, Ferretti R. Diamond-like carbon-gate pH-ISFET. Sensors and Actuators B: Chemical. 1997;8(1–3):441–445. [Google Scholar]
- Reinhoudt DNS, Ernst JR. The transduction of host-guest interactions into electronic signals by molecular systems. Adv Mater. 1990;8(1):23–32. doi: 10.1002/adma.19900020105. [DOI] [Google Scholar]
- Cobben PLHME, Bomer RJM, Bergveld JG, Piet V, Willem R, David N. Transduction of selective recognition of heavy metal ions by chemically modified field effect transistors (CHEMFETs) J Am Chem Soc. 1992;8(26):10573–10582. doi: 10.1021/ja00052a063. [DOI] [Google Scholar]
- Guth U, Gerlach F, Decker M, Oelßner W, Vonau W. Solid-state reference electrodes for potentiometric sensors. Journal of Solid State Electrochemistry. 2009;8(1):27–39. doi: 10.1007/s10008-008-0574-7. [DOI] [Google Scholar]
- Cadogan A, Gao Z, Lewenstam A, Ivaska A, Diamond D. All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal Chem. 1992;8(21):2496–2501. doi: 10.1021/ac00045a007. [DOI] [Google Scholar]
- Jiménez C, Bartroli J. Development of an ion-sensitive field effect transistor based on PVC membrane technology with improved long-term stability. Electroanalysis. 1997;8(4):316–319. doi: 10.1002/elan.1140090411. [DOI] [Google Scholar]
- Bratov A, Muñoz J, Dominguez C, Bartrolí J. Photocurable polymers applied as encapsulating materials for ISFET production. Sensors and Actuators B: Chemical. 1995;8(1–3):823–825. [Google Scholar]
- Kuang B, Mahmood HS, Quraishi MZ, Hoogmoed WB, Mouazen AM, van Henten EJ. In: Advances in Agronomy. Donald LS, editor. Waltham: Academic; 2012. Chapter four - sensing soil properties in the laboratory, in situ, and on-line: a review; pp. 155–223. [Google Scholar]
- Seymour RB. Plastics. Ind Eng Chem. 1966;8(8):61–73. doi: 10.1021/ie50680a011. [DOI] [Google Scholar]
- Cecilia JJJ, Orozco A, Baldi Q. ISFET based microsensors for environmental monitoring. Sensors (Basel, Switzerland) 2009;8(1):1. doi: 10.3390/s100100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WY, Cruz FRG, Szu H, Pijanowska DG, Dawgul M, Torbicz W, Grabiec PB, Jarosewicz B, Chiang J-L, Chang KC, Cheng C, Ho W-P. In: Independent Component Analyses, Wavelets, Neural Networks, Biosystems, and Nanoengineering VII. Szu HH, Agee FJ, editor. Bellingham: SPIE; 2009. ISFET electronic tongue system for environmental multi-ion sensing with independent component analysis signal processing; p. 73431D. [Google Scholar]
- Haigang Yang HS, Jinghong H, Jinbao W, Zengjin L, Shanhong X, Hua Z. Proceedings of the Fifth International Workshop on System-on-Chip for Real-Time Applications: Banff; July 20–24, 2005. Piscataway: IEEE Computer Society; 2005. A pH-ISFET based micro sensor system on chip using standard CMOS technology; pp. 180–183. [Google Scholar]
- Lee D, Cui T. pH-dependent conductance behaviors of layer-by-layer self-assembled carboxylated carbon nanotube multilayer thin-film sensors. J Vac Sci Technol. 2009;8(2):842. doi: 10.1116/1.3002386. [DOI] [Google Scholar]
- Martinoia S, Massobrio P. ISFET–neuron junction: circuit models and extracellular signal simulations. Biosens Bioelectron. 2004;8(11):1487–1496. doi: 10.1016/j.bios.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Bousse L, Bergveld P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sensors and Actuators. 1984;8(1):65–78. doi: 10.1016/0250-6874(84)80028-1. [DOI] [Google Scholar]
- Steinhoff G, Hermann M, Schaff WJ, Eastman LF, Stutzmann M, Eickhoff M. pH response of GaN surfaces and its application for pH-sensitive field-effect transistors. Appl Phys Lett. 2003;8(1):177–179. doi: 10.1063/1.1589188. [DOI] [Google Scholar]
- Pijanowska D, Torbicz W. Optoelectronic and Electronic Sensors II. Bellingham: SPIE; 1997. Simple method of enzyme immobilization for pH-ISFET-based urea biosensors; pp. 219–226. [Google Scholar]
- Jamasb S, Collins SD, Smith RL. Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 1997: Chicago; October 30-November 2, 1997. Piscataway: IEEE; 1997. Correction of instability in ion-selective field effect transistors (ISFETs) for accurate continuous monitoring of pH; pp. 2337–2340. [Google Scholar]
- Couto SR, Moldes D, Sanromán MA. Optimum stability conditions of pH and temperature for ligninase and manganese-dependent peroxidase from Phanerochaete chrysosporium. Application to in vitro decolorization of Poly R-478 by MnP. World J Microbiol Biotechnol. 2006;8(6):607–612. doi: 10.1007/s11274-005-9078-0. [DOI] [Google Scholar]
- Pokhrel S, Joo JC, Kim YH, Yoo YJ. Rational design of a Bacillus circulans xylanase by introducing charged residue to shift the pH optimum. Process Biochem. 2012;8(12):2487–2493. doi: 10.1016/j.procbio.2012.10.011. [DOI] [Google Scholar]
- Morgenshtein A, Sudakov-Boreysha L, Dinnar U, Jakobson CG, Nemirovsky Y. Wheatstone-Bridge readout interface for ISFET/REFET applications. Sens Actuators B Chem. 2004;8(1):18–27. doi: 10.1016/j.snb.2003.07.017. [DOI] [Google Scholar]
- Chen S, Zhang Z-B, Laipeng M, Ahlberg P, Gao X, Qui Z, Wu D, Ren W, Cheng H-M, Zhang S-L. A graphene field-effect capacitor sensor in electrolyte. Appl Phys Lett. 2012;8(15):154106–154105. doi: 10.1063/1.4759147. [DOI] [Google Scholar]
- Zhao Y, Song X, Song Q, Yin Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm. 2012;8(20):6710–6719. doi: 10.1039/c2ce25509j. [DOI] [Google Scholar]
- Adam S, Das Sarma S. Transport in suspended graphene. Solid State Communications. 2008;8(9–10):356–360. [Google Scholar]
- Datta S. Electronic Transport in Mesoscopic Systems. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Datta S. Quantum Transport: Atom to Transistor. New York: Cambridge University Press; 2005. [Google Scholar]
- Peres NMR, Castro Neto AH, Guinea F. Conductance quantization in mesoscopic graphene. Physical Review B. 2006. p. 195411.
- Moriconi L, Niemeyer D. Graphene conductivity near the charge neutral point. Physical Review B. 2011;8(19):193401. [Google Scholar]
- Fu W, Nef C, Knopfmacher O, Tarasov A, Weiss M, Calame M, Schönenberger C. Graphene transistors are insensitive to pH changes in solution. Nano Lett. 2011;8(9):3597–3600. doi: 10.1021/nl201332c. [DOI] [PubMed] [Google Scholar]
- Bonanni AL, Adeline Hulling Pumera M. Graphene for impedimetric biosensing. Trac-Trends in Analytical Chemistry. 2012;8:12–21. [Google Scholar]