Figure 2.
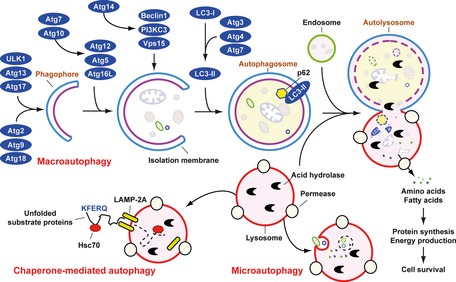
Means by which autophagy delivers antigen into the autolysosome. Microautophagy refers to the sequestration of cytosolic components directly by lysosomes through invaginations within their limiting membrane. Chaperone‐mediated autophagy involves direct translocation of unfolded substrate proteins (KFERQ‐like motif) across the lysosome membrane through the action of a cytosolic and lysosomal chaperone heat shock cognate protein of 70 kDa (Hsc70), and the integral membrane receptor lysosome‐associated membrane protein type 2A (LAMP‐2A). In the case of macroautophagy, the cargo is sequestered within a unique double membrane cytosolic vesicle, an autophagosome. The autophagosome itself is formed by expansion of the phagophore. The autophagosome undergoes fusion with a late endosome or lysosome to form an autolysosome, in which the sequestered material is degraded. Degradation of membrane lipids and proteins by the autolysosome generates free fatty acids, nucleotides, and amino acids that can be reused by the cell to maintain mitochondrial ATP energy production, protein synthesis, and thereby promote cell survival. The molecular machinery of macroautophagy was largely discovered in yeast and the centrally important proteins referred to as autophagy‐related (ATG) proteins although some similar proteins in mammals have disparate names (Beclin‐1 = ATG6, LC3 = ATG8 for example).
