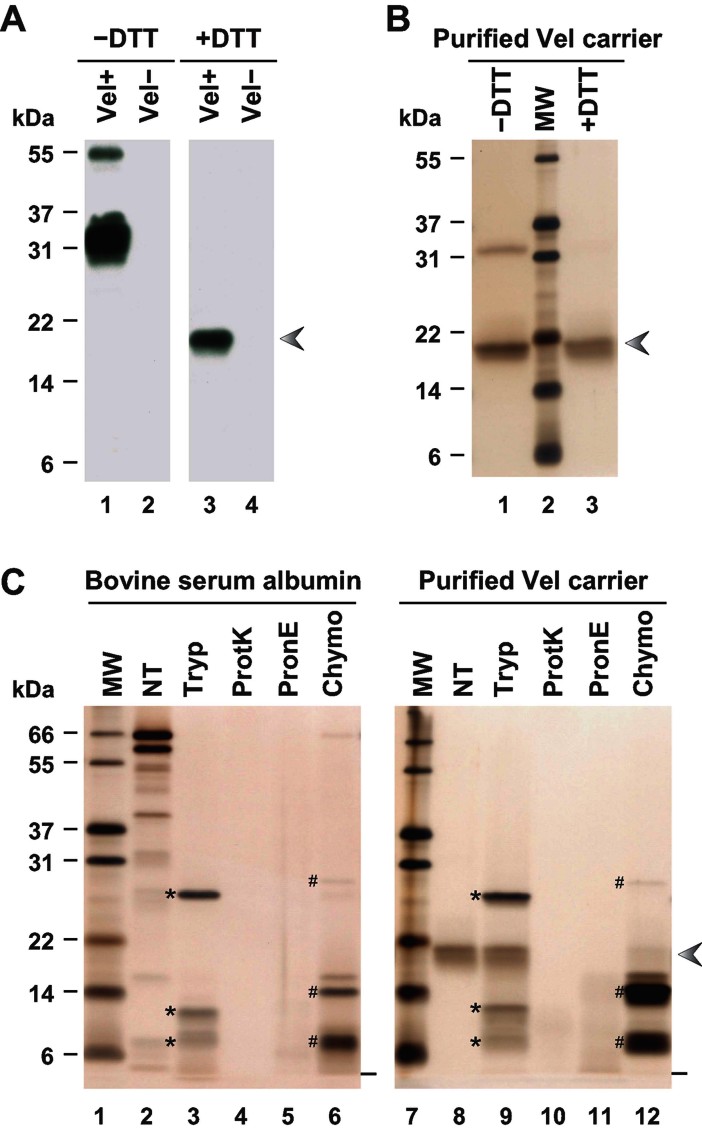
Figure 2.
Purification and protease sensitivity of the 18 kDa reduced form of the Vel antigen carrier.
A. RBC membrane extracts prepared from a Vel+ subject (lanes 1 and 3) and a Vel− subject (lanes 2 and 4) were resolved by SDS–PAGE under non-reducing (lanes 1 and 2) or reducing conditions (lanes 3 and 4), and probed with an anti-Vel.
B. The 18 kDa material purified from a Vel+ RBC membrane extract was analyzed by SDS–PAGE, under non-reducing (lane 1) or reducing conditions (lane 3), and detected by silver staining.
C. Bovine serum albumin (lanes 2–6) and the 18 kDa band purified from a Vel+ RBC membrane extract (lanes 8–12) were treated with trypsin (Tryp), proteinase K (ProtK), pronase E (PronE) or chymotrypsin (Chymo), or were left untreated (NT), and analyzed by reducing SDS–PAGE followed by silver staining. The electrophoretic migration fronts are indicated by dashes, trypsin and its autoproteolytic fragments by asterisks, and chymotrypsin and its autoproteolytic fragments by number signs. The arrowhead indicates the migration of the Vel antigen carrier under reducing conditions.