Figure 7.
Intranasal administration of PD1 or RvD1 promotes resolution of allergic airway inflammation.
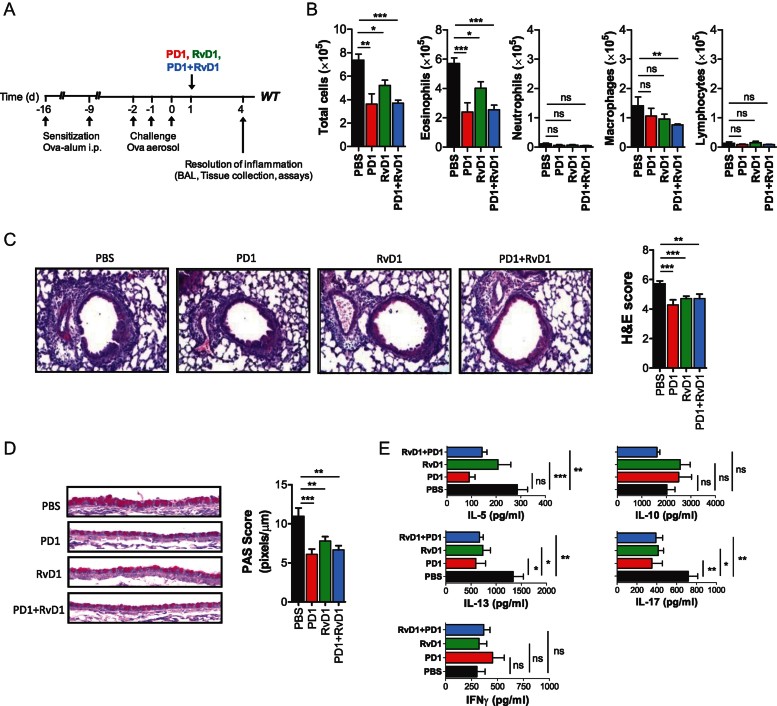
A. Protocol of PD1, RvD1, PD1 and RvD1 (100 ng/mouse) or vehicle (PBS) administration in wild-type mice.
B. Total and differential cell counts in BALF of OVA sensitized and challenged (OVA/OVA) mice at Day 4 post-challenge. Results are expressed as mean ± SEM of 9 mice per group.
C. Histological assessment of lung inflammation at Day 4 post-challenge. Haematoxylin and eosin (H&E)-stained lung sections and histological scoring expressed as mean values ± SEM from 9 mice/group are shown.
D. Histological assessment of mucus secretion at Day 4 post-challenge. Periodic acid Schiff (PAS)-stained sections and morphometric analysis expressed as mean values ± SEM from 9 mice/group are shown.
E. Allergen-specific effector T cell responses in mediastinal LNs at Day 4 post-challenge. Cytokine levels are expressed as mean values ± SEM in supernatants of OVA-stimulated mediastinal LN cultures of 9 mice per group. *p < 0.05, **p < 0.01, ***p < .001, ns: non-significant compared to vehicle-treated control.