Abstract
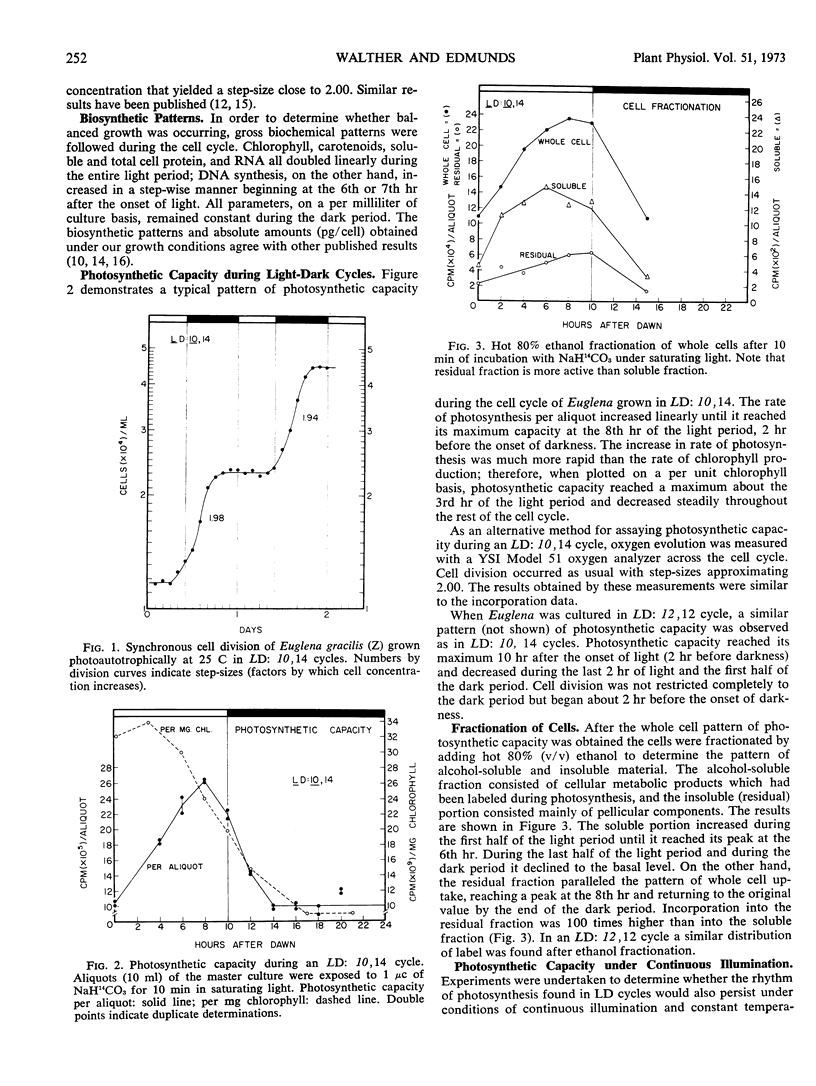
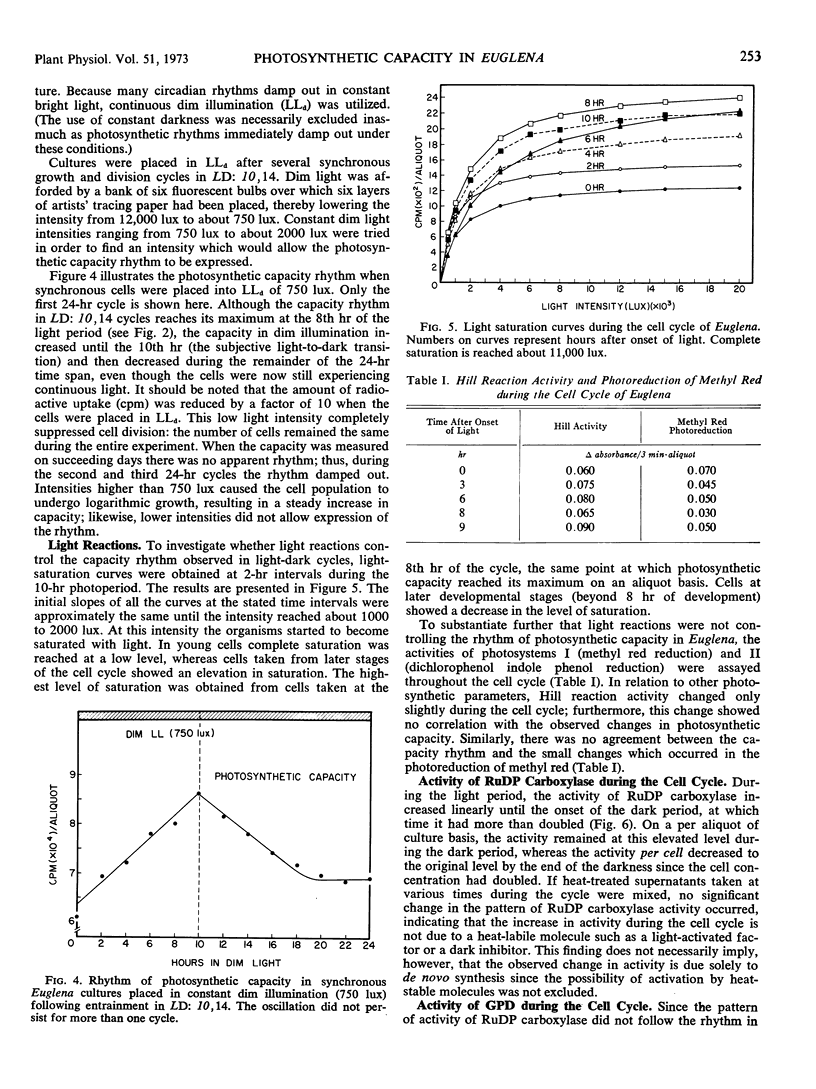
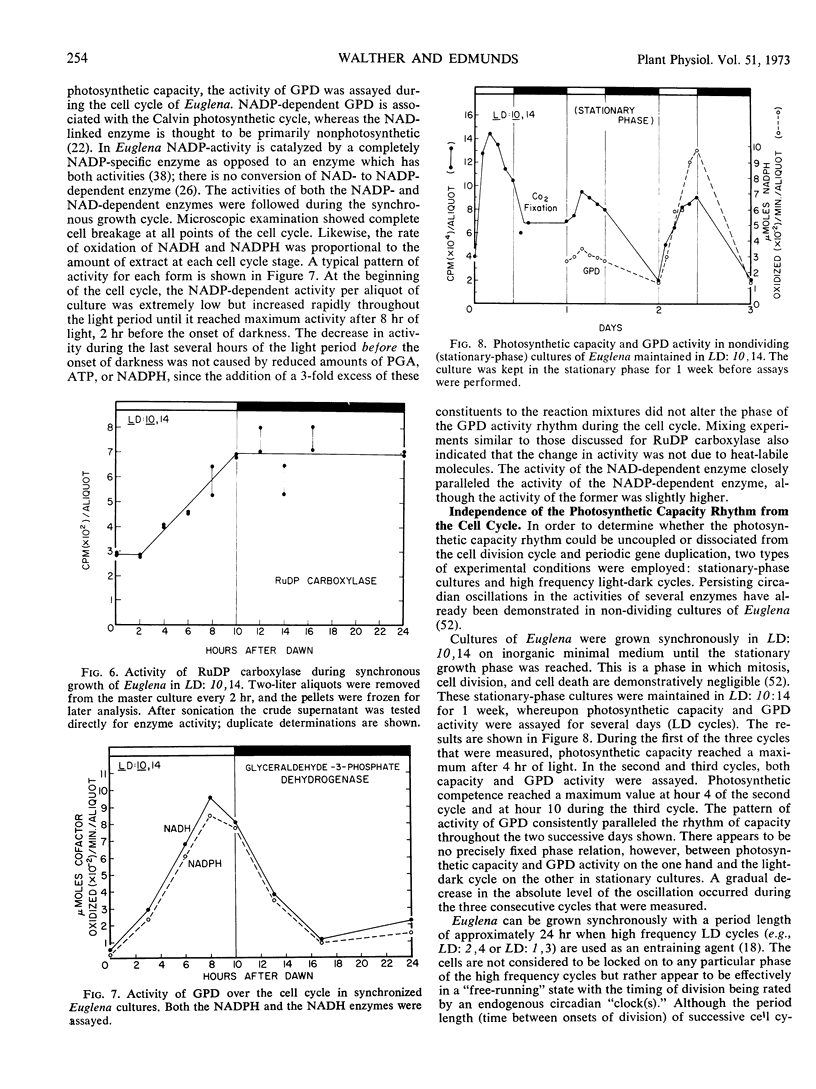
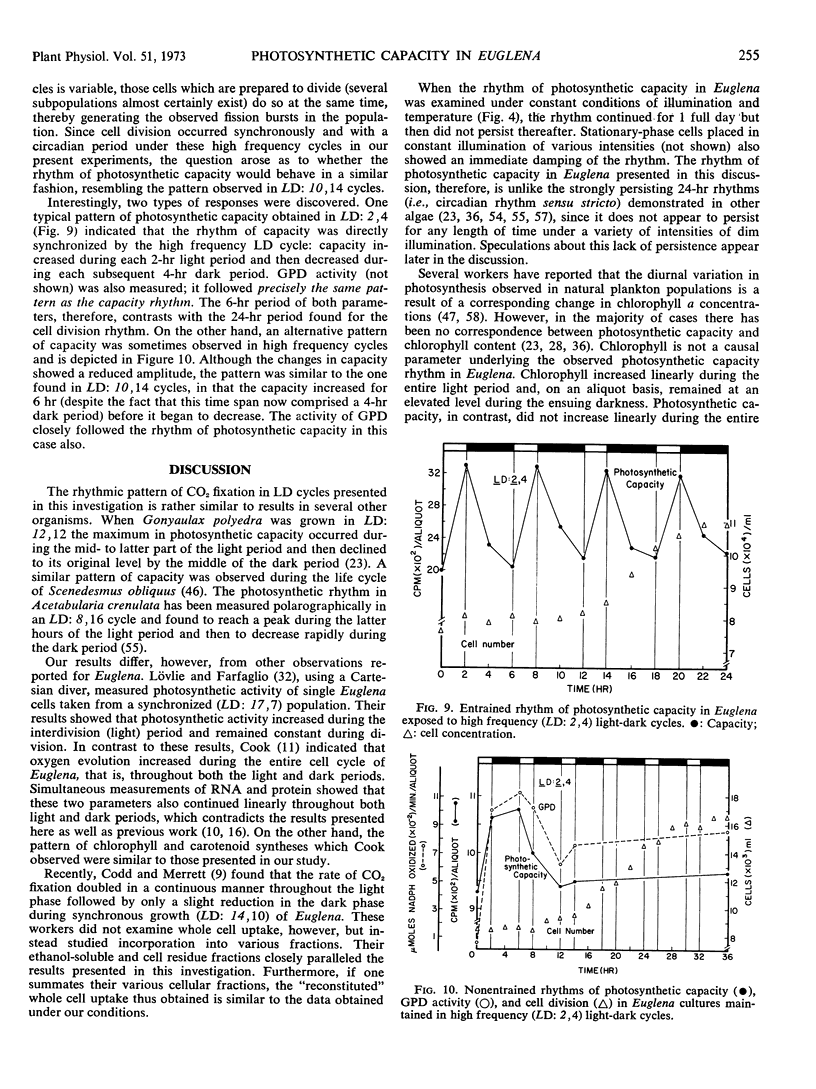
Synchronous cell division in Euglena gracilis (strain Z) was obtained in 24-hour light cycles consisting of 10 hours of light and 14 hours of darkness; cell division was restricted to the dark period. Photosynthetic capacity was found to vary in a cyclic manner during the cell cycle, reaching a peak 2 hours before the onset of darkness. Light reactions were investigated during the cell cycle to determine what role they played in the control of the observed rhythmic changes in capacity. Light-saturation curves showed no major change in the light-limited region. No fluctuations were found in Hill reaction activity or photoreduction of methyl red during the cell cycle. These results imply that the reactions comprising photosystems I and II do not generate the capacity rhythm.
Some of the photosynthetic dark reactions were also followed during the cell cycle in an attempt to determine their possible role in the control of the rhythm of photosynthetic capacity. The activity of ribulose-1, 5-diphosphate carboxylase showed no correlation with the rhythm. On the other hand, the activity of glyceraldehyde-3-phosphate dehydrogenase was found to parallel the change in photosynthetic rate under various growth conditions. The rhythm in photosynthetic capacity could be effectively divorced from the cell cycle itself by placing cultures in high frequency light cycles (LD: 2,4) or in stationary growth-phase conditions. If synchronously dividing cultures previously grown in LD: 10, 14 were released into continuous dim illumination and constant temperature, the rhythm of capacity persisted for only one full cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amotz A., Avron M. Is nicotinamide adenine dinucleotide phosphate an obligatory intermediate in photosynthesis? Plant Physiol. 1972 Feb;49(2):244–248. doi: 10.1104/pp.49.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce V. G., Pittendrigh C. S. TEMPERATURE INDEPENDENCE IN A UNICELLULAR "CLOCK". Proc Natl Acad Sci U S A. 1956 Sep;42(9):676–682. doi: 10.1073/pnas.42.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Bush K. J., Sweeney B. M. The Activity of Ribulose Diphosphate Carboxylase in Extracts of Gonyaulax polyedra in the Day and the Night Phases of the Circadian Rhythm of Photosynthesis. Plant Physiol. 1972 Oct;50(4):446–451. doi: 10.1104/pp.50.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK J. R., JAMES T. W. Light-induced division synchrony in Euglena gracills var. bacillaris. Exp Cell Res. 1960 Dec;21:583–589. doi: 10.1016/0014-4827(60)90292-5. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. Photosynthetic products of division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):635–639. doi: 10.1104/pp.47.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMUNDS L. N., Jr REPLICATION OF DNA AND CELL DIVISION IN SYNCHRONOUSLY DIVIDING CULTURES OF EUGLENA GRACILIS. Science. 1964 Jul 17;145(3629):266–268. doi: 10.1126/science.145.3629.266. [DOI] [PubMed] [Google Scholar]
- Edmunds L. N., Jr, Funch R. R. Circadian rhythm of cell division in Euglena: effects of random illumination regimen. Science. 1969 Aug 1;165(3892):500–503. doi: 10.1126/science.165.3892.500. [DOI] [PubMed] [Google Scholar]
- Edmunds L. N., Jr Studies on synchronously dividing cultures of Euglena gracilis Klebs (strain Z). 3. Circadian components of cell division. J Cell Physiol. 1966 Feb;67(1):35–43. doi: 10.1002/jcp.1040670105. [DOI] [PubMed] [Google Scholar]
- Feldman J. F. Circadian rhythmicity in amino acid incorporation in Euglena gracilis. Science. 1968 Jun 28;160(3835):1454–1456. doi: 10.1126/science.160.3835.1454. [DOI] [PubMed] [Google Scholar]
- Fuller R. C., Gibbs M. Intracellular and Phylogenetic Distribution of Ribulose 1,5-Diphosphate Carboxylase and D-Glyceraldehyde-3-Phosphate Dehydrogenases. Plant Physiol. 1959 May;34(3):324–329. doi: 10.1104/pp.34.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., ASTRACHAN L., SWEENEY B. M. A persistent daily rhythm in photosynthesis. J Gen Physiol. 1961 Sep;45:69–76. doi: 10.1085/jgp.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Hudock G. A., Fuller R. C. Control of Triosephosphate Dehydrogenase in Photosynthesis. Plant Physiol. 1965 Nov;40(6):1205–1211. doi: 10.1104/pp.40.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett R. M., Edmunds L. N., Jr Persisting circadian rhythm of cell division in a photosynthetic mutant of Euglena. Science. 1970 Mar 27;167(3926):1730–1733. doi: 10.1126/science.167.3926.1730. [DOI] [PubMed] [Google Scholar]
- Kirk J. T., Allen R. L. Dependence of chloroplast pigment synthesis on protein synthesis: effect of actidione. Biochem Biophys Res Commun. 1965 Dec 21;21(6):523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- Lövlie A., Farfaglio G. Increase in photosynthesis during the cell cycle of Euglena gracilis. Exp Cell Res. 1965 Sep;39(2):418–434. doi: 10.1016/0014-4827(65)90045-5. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Pupillo P., Baccarini-Melandri A. D-glyceraldehyde-3-phosphate dehydrogenase in photosynthetic cells. I. The reversible light-induced activation in vivo of NADP-dependent enzyme and its relationship to NAD-dependent activities. Biochim Biophys Acta. 1970 Nov 11;220(2):178–189. doi: 10.1016/0005-2744(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Müller B. On the mechanism of the light-induced activation of the NADP-dependent glyceraldehyde phosphate dehydrogenase. Biochim Biophys Acta. 1970 Apr 7;205(1):102–109. doi: 10.1016/0005-2728(70)90066-6. [DOI] [PubMed] [Google Scholar]
- PALMER J. D., LIVINGSTON L., ZUSY D. A PERSISTENT DIURNAL RHYTHM IN PHOTOSYNTHETIC CAPACITY. Nature. 1964 Sep 5;203:1087–1088. doi: 10.1038/2031087a0. [DOI] [PubMed] [Google Scholar]
- PERINI F., KAMEN M. D., SCHIFF J. A. IRON-CONTAINING PROTEINS IN EUGLENA. I. DETECTION AND CHARACTERIZATION. Biochim Biophys Acta. 1964 Jul 29;88:74–90. doi: 10.1016/0926-6577(64)90155-x. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Lyman H. Isolation of mutants of Euglena gracilis with impaired photosynthesis. Plant Physiol. 1968 Aug;43(8):1284–1290. doi: 10.1104/pp.43.8.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOROKIN C. PHOTOSYNTHESIS IN CELL DEVELOPMENT. Biochim Biophys Acta. 1965 Jan 25;94:42–52. doi: 10.1016/0926-6585(65)90006-3. [DOI] [PubMed] [Google Scholar]
- Schor S., Siekevitz P., Palade G. E. Cyclic Changes in Thylakoid Membranes of Synchronized Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1970 May;66(1):174–180. doi: 10.1073/pnas.66.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger H., Bishop N. I. Quantum yield of photosynthesis in synchronous Scenedesmus cultures. Nature. 1967 Apr 8;214(5084):140–142. doi: 10.1038/214140a0. [DOI] [PubMed] [Google Scholar]
- Sulzman F. M., Edmunds L. N., Jr Persisting circadian oscillations in enzyme activity in non-dividing cultures of Euglena. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1338–1344. doi: 10.1016/0006-291x(72)90219-7. [DOI] [PubMed] [Google Scholar]
- Sweeney B. M., Haxo F. T. Persistence of a Photosynthetic Rhythm in Enucleated Acetabularia. Science. 1961 Oct 27;134(3487):1361–1363. doi: 10.1126/science.134.3487.1361. [DOI] [PubMed] [Google Scholar]
- Vanden Driessche T. Circadian rhythms in Acetabularia: photosynthetic capacity and chloroplast shape. Exp Cell Res. 1966 Apr;42(1):18–30. doi: 10.1016/0014-4827(66)90315-6. [DOI] [PubMed] [Google Scholar]


