Abstract
Background and Aims
Orchid mycorrhizas exhibit a unique type of mycorrhizal symbiosis that occurs between fungi and plants of the family Orchidaceae. In general, the roots of orchids are typically coarse compared with those of other plant species, leading to a considerably low surface area to volume ratio. As a result, orchids are often ill-adapted for direct nutrient acquisition from the soil and so mycorrhizal assocaitions are important. However, the role of the fungal partners in the acquisition of inorganic and organic N by terrestrial orchids has yet to be clarified.
Methods
Inorganic and amino acid N uptake by non-mycorrhizal and mycorrhizal Cymbidium goeringii seedlings, which were grown in pots in a greenhouse, was investigated using a 15N-labelling technique in which the tracer was injected at two different soil depths, 2·5 cm or 7·5 cm. Mycorrhizal C. goeringii seedlings were obtained by inoculation with three different mycorrhizal strains isolated from the roots of wild terrestrial orchids (two C. goeringii and one C. sinense).
Key Results
Non-mycorrhizal C. goeringii primarily took up NO3− from tracers injected at 2·5-cm soil depth, whereas C. goeringii inoculated with all three mycorrhiza primarily took up NH4+ injected at the same depth. Inoculation of the mycorrhizal strain MLX102 (isolated from adult C. sinense) on C. goeringii roots only significantly increased the below-ground biomass of the C. goeringii; however, it enhanced 15NH4+ uptake by C. goeringii at 2·5-cm soil depth. Compared to the uptake of tracers injected at 2·5-cm soil depth, the MLX102 fungal strain strongly enhanced glycine-N uptake by C. goeringii from tracers injected at 7·5-cm soil depth. Cymbidium goeringii inoculated with CLB113 and MLX102 fungal strains demonstrated a similar N uptake pattern to tracers injected at 2·5-cm soil depth.
Conclusions
These findings demonstrate that mycorrhizal fungi are able to switch the primary N source uptake of a terrestrial orchid, in this case C. goeringii, from NO3− to NH4+. The reasons for variation in N uptake in the different soil layers may be due to possible differentiation in the mycorrhizal hyphae of the C. goeringii fungal partner.
Keywords: Mycorrhizal fungi, amino acid uptake, Cymbidium species, nutrient acquisition, 15N labelling
INTRODUCTION
Nitrogen (N) is a major element limiting plant growth in most terrestrial ecosystems (LeBauer and Treseder, 2008). A large amount of N is stored in soil organic matter; however, the traditional concept of N cycling assumes that plants only take up inorganic N (i.e. NH4+ and NO3−) in soils, relying on soil microorganisms to unlock N from organic forms (Schimel and Bennett, 2004; Rothstein, 2009). However, some plants, including those that do not have mycorrhization, have the capacity to directly utilize free low molecular-weight organic N compounds (i.e. amino acids and small peptides) from soils (Chapin et al., 1993; Marschner, 1995; Raab et al., 1996; Thornton, 2001), while other plants rely on specialized symbiotic associations to exploit organic N sources (Read, 1991). The mechanisms of how arbuscular, ecto- and ericoid mycorrhizas influence the N nutrition of plants have been well documented (George et al., 1995; Read and Perez-Moreno, 2003; Finlay, 2005; Hodge and Fitter, 2010; Neumann and George, 2010; Miransari, 2011; Veresoglou et al., 2012); however, the roles of orchid mycorrhizas in N nutrition are less well explored.
Orchid mycorrhizas exhibit a unique type of mycorrhizal symbiosis that occurs between fungi and plants of the family Orchidaceae (Smith and Read, 2008; Imhof, 2009). In general, the roots of orchids are typically coarse compared with those of other plant species, leading to a considerably low surface area to volume ratio. As a result, orchids are often ill-adapted for direct nutrient acquisition from the soil (Leake, 1994). Numerous studies have demonstrated that mycorrhizal fungi strongly enhance nutrient acquisition by orchids in their native ecosystems (Alexander, 2007; Smith and Read, 2008). All terrestrial orchids investigated to date depend on a mycorrhizal partnership to provide nutrients for germination (Rasmussen, 1995; Cameron et al., 2006; Rasmussen and Rasmussen, 2009). At the achlorophyllous protocorm stage, carbon and nutrient acquisition by orchids is completely dependent on the fungus partner (Rasmussen and Rasmussen, 2009). Even after photosynthesis is established, most orchid species remain, to some extent, reliant on the fungus for carbon and nutrients (Cameron et al., 2006; Rasmussen and Rasmussen, 2009). For example, adult mycorrhizal Goodyera repens acquires 100 times more phosphorus than non-mycorrhizal controls (Alexander et al., 1984). Subsequent studies further demonstrated that phosphorus and glycine-N maybe transferred from the mycorrhizal fungus to a terrestrial orchid, such as Goodyera repens (Cameron et al., 2006, 2007). Nevertheless, it still remains unclear how fungal partners influence inorganic and organic N acquisition by terrestrial orchids (Alexander, 2007; Smith and Read, 2008).
The genus Cymbidium is widely distributed from east and south-east Asia to Australia (DuPuy and Cribb, 1988; Berg-Pana, 2005; DuPuy and Cribb, 2007). Among Cymbidium species, C. goeringii is an important terrestrial orchid that is native to south-western and north-eastern China. To investigate the role of the fungal partner in organic and inorganic N acquisition by C. goeringii, we isolated three mycorrhizal fungal strains from wild Cymbidium species in Yunnan Province, China. Two fungal strains (CLB111 and CLB113) were isolated from the host C. goeringii, which was collected from the Baoshan region of south-western Yunnan. The third fungal strain (MLX102) was isolated from the host C. sinense, which was collected from the Xishuangbanna region of southern Yunnan. All three strains were identified as Rhizoctonia based on morphological and molecular techniques. Specifically, CLB113 and MLX102 strains are phylogenetically more closely related, while CLB111 and CLB113 strains are more distantly related (Wu et al., 2010). Non-mycorrhizal C. goeringii seedlings were obtained through culture techniques (Wu et al., 2010). After these seedlings were grown in a greenhouse, the isolated fungi were inoculated back onto their roots. Using these non-mycorrhizal and mycorrhizal C. goeringii seedlings, we tested the following hypotheses: (a) mycorrhizal fungi cause plant hosts to switch primary soil N source uptake, with the specific pattern of uptake varying according to the mycorrhizal fungi plant source; and (b) while plants have fewer roots in deeper soils, the extraradical mycelium of the mycorrhizas facilitates the uptake of inorganic and organic N from deep soil by orchids.
MATERIALS AND METHODS
Non-mycorrhizal Cymbidium seedlings
Ripe seed capsules of Cymbidium goeringii were collected from a subtropical forest in the Baoshan region (22°49′N, 103°36′E, 1980 m a.s.l.) of Yunnan Province in south-western China, where the dominant trees were a broad-leaved evergreen species (Quercus acutissima) and a coniferous species (Pinus yunnanensis). The annual mean temperature of this region is approx. 16 °C and the annual mean precipitation is 1447 mm in the last 10 years of the 20th century.
Non-mycorrhizal seedlings of C. goeringii were obtained by Wu et al. (2010). In brief, both germination and seedling growth stages were incubated at 25 °C with a 12 : 12 h light : dark cycle under fluorescent lamps (40 mmol m−2 s−1). After approx. 30 d, the seedlings were transplanted to pots (16 cm diameter; 15 cm height) containing moss, vermiculite, and sand in a ratio of 1 : 1 : 1 (v/v/v), which had been autoclaved at 121 °C for 60 min to eliminate any microorganisms from the substrates.
Fungal strain isolation
To obtain mycorrhizal fungal strains from Cymbidium plants, we collected three Cymbidium plants representing three plant species native to Yunnan Province. Two C. goeringii plants were collected from the Baoshan region of south-western Yunnan (referred to as the CLB111 and CLB113 fungal strains); one C. sinense plant was collected from the Xishuangbanna region in southern Yunnan (referred to as the MLX102 fungal strain). Mycorrhizal fungi were isolated from the collected roots according to the method described by Warcup and Talbot (1967), with minor modifications. Detailed information, including modifications, is provided by Wu et al. (2010). The cultures were observed under a light microscope (×400), and putatively identified based on the criteria established for other orchid mycorrhizal fungi based on hyphal morphology from cultures (Sneh et al., 1991; Currah et al., 1997).
Establishing symbiotic associations between C. goeringii seedlings and mycorrhizal fungi
The transplanted C. goeringii seedlings were grown in the greenhouse at 25 °C, 70–80 % humidity, with natural light–dark cycles (Kunming City, China). After 1 month of growth in the greenhouse, the seedlings of C. goeringii were inoculated with mycelial cultures of the three specific fungal strains: CLB111, CLB113 and MLX102. The fungal mycelial cultures were obtained by growing specific fungal strains on sterilized Q. acutissima leaf medium at 25 °C in the dark for 2 months. Quercus acutissima leaves containing fungal mycelia were then buried as inocula about 1 cm away from the C. goeringii seedlings, at the stage where they had one leaf per seedling. Thirty seedlings were inoculated for each fungal strain (referred to as the mycorrhizal group). For the control, 30 seedlings were inoculated with sterilised Q. acutissima leaf medium that was clean of any fungal strains, and were also buried about 1 cm away from the C. goeringii seedlings (referred to as the non-mycorrhizal group).
Approximately 6 months after fungal inoculation, the presence of mycorrhizal symbiosis with the seedlings was examined (Fig. 1). This was completed for the inoculated greenhouse orchids, following the protocol described for wild orchids (Warcup and Talbot, 1967). As expected, fungi were not isolated from the non-inoculated orchids (Fig. 2). The fungal strains re-isolated from the inoculated plants were compared with the original inoculated fungal strains using random amplified polymorphic DNA (RAPD) markers and internal transcribed spacers (ITS) sequencing. The results showed that the re-isolated strains were genetically identical to the originally inoculated strains (Wu et al., 2010).
Fig. 1.

Growth performance of Cymbidium goeringii seedlings with and without mycorrhizal fungal inoculation at 6 months after inoculation. From left to right: seedling without mycorrhizal fungal inoculation (Control), seedling inoculated with the MLX102, CLB113 and CLB111 fungal strains. Scale bar as indicated on the figure.
Fig. 2.
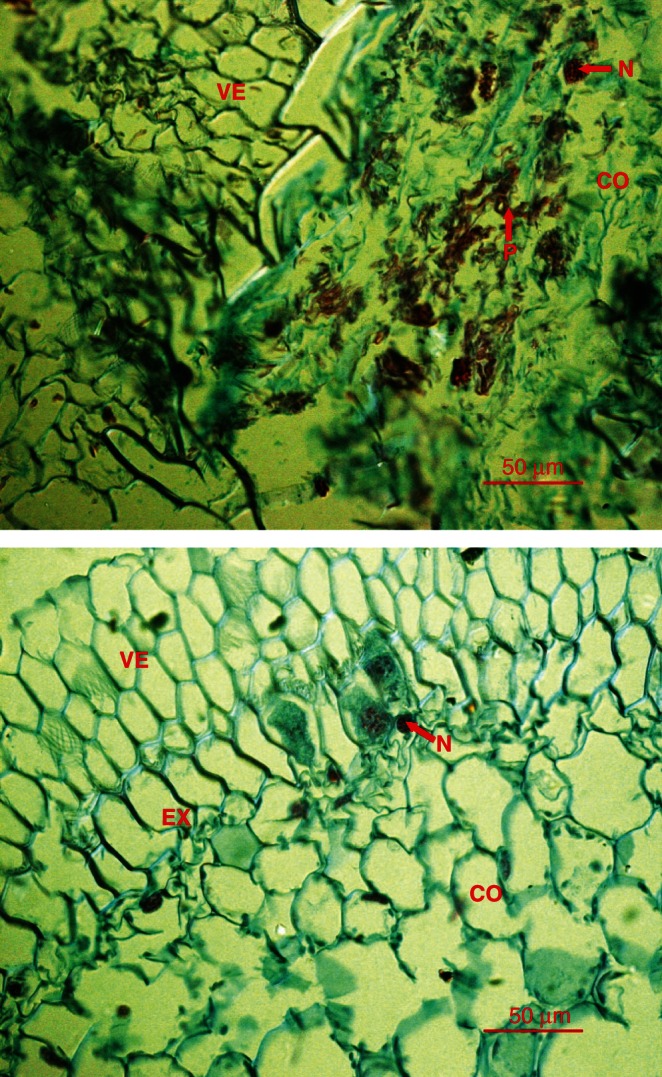
Microstructure of the mycorrhizas of C. goeringii 6 months after inoculation with strain CLB111 (upper), and the uninoculated control (lower). The letters in the images indicate root structures: CO, cortex; N, nucleus; P, peloton; PI, pith; VE, velamen; EX, exodermis. Red arrows point to the nucleus and peloton.
15N labelling
Six months after fungal inoculation, we injected 15N-labelled KNO3 (99·3 % 15N enrichment), (NH4)2SO4 (99·5 % 15N enrichment) or glycine (98·4 % 15N enrichment) into the soils of mycorrhizal and non-mycorrhizal C. goeringii seedlings. The tracers were injected in a pattern representing the three points of a triangle, with 5 cm length between points and the seedling at the centre of the triangle. At each point with 1 mL of tracer was injected. Nitrogen was applied at 1 µg N g−1 substrate. The tracers were injected at 2·5-cm and 7·5-cm soil depths. Reference pots (i.e. controls), which were not labelled with 15N tracer, were supplied with the equivalent amount of water. Therefore, a three-factor design was constructed: three mycorrhizal species (CLB111, CLB113 and MLX102) with non-mycorrhizal seedlings as the control, two injection depths (2·5 cm and 7·5 cm) and three N species (15NO3−, 15NH4+ and 15N-glycine). Four replicates per treatment were established. Plants were collected 24 h after 15N labelling. Roots were first rinsed with tap water, and were then immersed in 0·5 mm CaCl2 for 30 min. Subsequently, the roots were rinsed with distilled water. Above-ground parts and roots were dried in an oven at 75 °C for 48 h. Dried plant materials were weighed and ground into a powder using a ball miller (MM200; Haan, Retsch, Germany) for N and 15N/14N measurements.
Analysis of 15N/14N in plant materials
Aliquots of plant material samples were weighed into tin capsules to analyse total N and15N/14N by continuous-flow gas isotope ratio mass spectrometry, which consists of an elemental analyser (EA 1110; CE Instruments, Milan, Italy), a ConFlo III device (Finnigan MAT, Bremen, Germany) and a gas isotope ratio mass spectrometer (MAT253; Finnigan MAT). Atmospheric N2 was used as the elemental reference material. The standard deviation of repeated measurements of laboratory standards was ±0·15 ‰.
Calculations and statistics
Atom % excess 15N was calculated as the atom % 15N difference between the same plant species from 15N-treated and control pots. 15N uptake by plants was calculated by multiplying the atom % excess and N content by the biomass. Because N was taken up by roots, the rates of 15N uptake by plants were calculated as 15N uptake by plants divided by root biomass and the time (24 h), expressed as μg N g−1 d. wt root d−1. Variation is represented by the standard errors of the means in the figures. A Dunnett's test was performed with an SPSS 20·0 software package (SPSS Inc., Chicago, IL, USA) to compare the effects of different fungal inoculation on seedling growth and N uptake rates with the non-mycorrhizal group. A paired t-test was used to compare the difference between different fungal inoculations. All differences were tested for significance at α = 0·05.
RESULTS
Effects of the fungus partner on the above- and below-ground biomass of C. goeringii
Inoculation of mycorrhizal fungi on the plant roots increased the above- and below-ground biomass of C. goeringii; however, biomass levels varied with respect to mycorrhizal species (Fig. 3). Six months after inoculation, the above- and below-ground biomass of non-mycorrhizal C. goeringii individuals averaged 0·12 ± 0·02 g pot−1 and 0·09 ± 0·01 g pot−1, respectively. Inoculation with CLB111 and CLB113 strains significantly increased the above- and below-ground biomass of C. goeringii. In contrast, the strain MLX102 isolated from another Cymbidium species significantly increased below-ground biomass of C. goeringii only. Among the three mycorrhizal fungi treatments, a significant difference in root biomass was observed between CLB111 and MLX102 treatments (Fig. 3). The root : shoot ratio of non-mycorrhizal C. goeringii seedlings was approx. 0·78 ± 0·08. Furthermore, while mycorrhizal fungi increased the root : shoot ratios, these values were <1 and not significantly different to the control (Fig. 3).
Fig. 3.
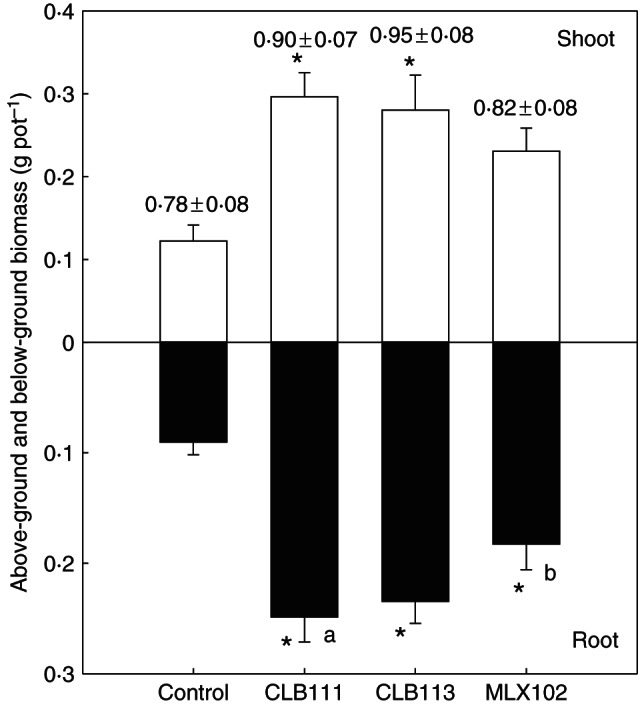
Effects of different mycorrhizal inoculations on the above- and below-ground biomass of orchid seedlings 6 months after inoculation (means and s.e.). The asterisk indicates a significant difference between the mycorrhizal fungi treatment (mycorrhizal seedlings) and the control treatment (non-mycorrhizal seedlings). Different letters indicates a significant difference between the mycorrhizal fungi treatments. The values above each column are the root to shoot ratios: there are no any significant differences between the control and the treatments inoculated with mycorrhizal fungi.
Effects of the fungal partner on inorganic and organic N uptake by C. goeringii
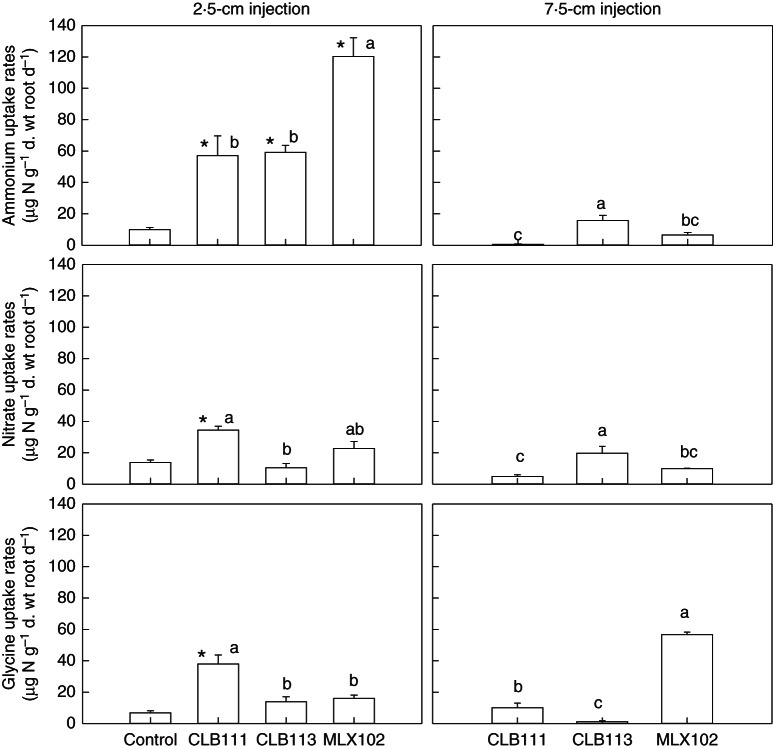
Non-mycorrhizal C. goeringii seedlings primarily took up 15NO3− from tracers injected at 2·5-cm soil depth, at a rate of 13·8 ± 1·6 µg N g−1 d. wt root d−1. All three mycorrhizal fungi, particularly MLX102, strongly enhanced 15NH4+ uptake by the roots from tracers injected at 2·5-cm soil depth (Fig. 4). In comparison, the fungal strain CLB111 significantly increased 15NO3− and 15N-glycine uptake by C. goeringii seedlings from tracers injected at 2·5-cm soil depth, while both CLB113 and MLX102 did not significantly alter the uptake of 15NO3− and 15N-glycine (Fig. 4).
Fig. 4.
Effects of different mycorrhizal inoculations on the inorganic and organic nitrogen uptake rates of orchid seedlings (6 months after inoculation) after tracers were injected at 2·5-cm (left) and 7·5-cm (right) soil depths. The values (means and s.e.) of four replicates are presented. Asterisks indicate a significant difference between the mycorrhizal fungi treatment (mycorrhizal seedlings) and the control treatment (non-mycorrhizal seedlings). Different letters indicate a significant difference between the mycorrhizal fungi treatments. These data represent the total uptake by the plant (above- and below-ground parts, including the endophyte), calculated per root d. wt per day.
Insufficient non-mycorrhizal C. goeringii seedlings were available to infer N uptake from tracers injected at 7·5-cm soil depth; however, we could determine N uptake from this layer for C. goeringii seedlings inoculated with the three mycorrhizal fungi. Compared with uptake of tracers injected at 2·5-cm soil depth, inoculation of the three mycorrhizal fungi significantly decreased the uptake rates of inorganic and organic N from tracers injected at 7·5-cm soil depth, with two exceptions. First, the fungus CLB113 facilitated the acquisition of more 15NO3− by C. goeringii seedlings from tracers injected at 7·5-cm soil depth compared with tracers injected at 2·5-cm soil depth. Secondly, the organic N uptake rate of the fungus MLX102 was approximately three times greater from tracers injected at 7·5-cm soil depth compared with tracers injected at 2·5-cm soil depth (Fig. 4).
For non-mycorrhizal C. goeringii seedlings, NO3− and glycine-N uptake contributed to 45·2 ± 6·4 % and 22·4 ± 4·9 % of total N uptake. Fungal CLB111 significantly decreased the contribution of NO3− to total N uptake, but increased NH4+ and glycine uptake by 35·9 % and 15·5 %, respectively, compared with the control. For the other two mycorrhizal fungal inoculations, NH4+ uptake contributed to >70 % of the total N uptake, whereas the contributions of glycine and NO3− were similar (Fig. 4).
DISCUSSION
In this study, we provide clear evidence that mycorrhizal fungi are able to switch the N source uptake of a terrestrial orchid C. goeringii from NO3− to NH4+ after the injection of tracers at 2·5-cm soil depth.
A large number of studies have shown that some epiphytic and terrestrial orchids have the capacity to take up organic N directly from soils in the form of free amino acids (Majerowicz et al., 2000; Majerowicz and Kerbauy, 2002; Cameron et al., 2006). In this study, we found that non-mycorrhizal C. goeringii seedlings are able to take up glycine-N; however, of the three tested fungal strains, only the fungal strain CLB111 significantly increased glycine-N uptake by mycorrhizal seedlings from tracers injected at 2·5-cm soil depth. We did not measure the N uptake of roots from tracers injected at 7·5-cm soil depth, because some of these non-mycorrhizal seedlings died. As the roots of non-mycorrhizal C. goeringii seedlings were generally short and concentrated in the top 5-cm soil layer (Fig. 1; Wu et al., 2010), we therefore assumed that the amount of N uptake by non-mycorrhizal C. goeringii seedlings from tracers injected at 7·5-cm soil depth was negligible. Accordingly, the fungal strains CLB111 and MLX102 strongly enhanced glycine-N uptake by C. goeringii seedlings from the deeper soil layer, whereas the CLB113 strain greatly increased NH4+ and NO3− uptake. This observation clearly reflects that mycorrhizal seedlings take up N forms differently at different soil depths. A possible explanation for this phenomenon is that there is differentiation in the hyphae of the tested mycorrhizal fungus. For instance, hyphae in the upper soil layer might be responsible for NH4+ uptake whereas hyphae in the deeper soil layer might be responsible for organic N uptake. This differentiation in hyphae functioning of ectomycorrhizal fungi has been previously suggested, and possibly represents different exploration strategies (Agerer, 2001). However, most of the literature on the exploration strategies of mycorrhizal fungi focuses on ectomycorrhizal fungi, with the functional significance of this strategy in relation to orchid mycorrhizas requiring further study.
The fungus MLX102 strongly increased 15NH4+ uptake by C. goeringii seedlings from tracers injected at 2·5-cm soil depth; however, it only significantly increased the below-ground biomass of roots compared with non-mycorrhizal seedlings (Fig. 3). This result might be ascribed to a mutualistic mechanism between the fungus partner and its host. For instance, more photosynthates might be transferred from the terrestrial orchid G. repens to its fungal partner, as a form of nutrient exchange (Cameron et al., 2006). The MLX102 strain strongly enhanced glycine-N uptake by C. goeringii seedlings from tracers injected at 7·5-cm soil depth; however, it did not alter glycine-N uptake from tracers injected at 2·5-cm soil depth. The difference in glycine-N uptake between the two soil depths might be related to different limitations on the fungal hyphae at different soil depths. For instance, hyphae might obtain more carbon from the rhizosphere in the top soil layer, but are lacking in N. In contrast, hyphae at deeper soil depths might be more carbon limited. Compared with the fungus strain MLX102, the other two strains isolated from the roots of C. goeringii plants showed different patterns of uptake. Although both strains increased the above- and below-ground biomass of C. goeringii seedlings, the CLB111 strain significantly enhanced the uptake of all three N forms, whereas the CLB113 strain only stimulated the uptake of NH4+ by C. goeringii seedlings from tracers injected at 2·5-cm soil depth (Fig. 4). This difference indicates that N acquisition by C. goeringii seedlings might be dependent on the mycorrhizal fungal species that is present.
Many studies have shown that NO3− is generally the preferred N form of tropical plants (Trépanier and Lamy, 2009). For example, some tropical epiphytic orchids prefer NO3- uptake (Wang, 2008; Trépanier and Lamy, 2009). In this study, we observed that non-mycorrhizal seedlings preferentially took up 15NO3−, whereas mycorrhizal seedlings preferentially took up NH4+ from tracers injected at 2·5-cm soil depth. Although asymbiotic orchids are not common in nature, our results clearly show that mycorrhizal fungi switched the uptake of the orchid C. goeringii from NO3− to NH4+ in the top soil. This trend is similar to that observed in ectomycorrhizal fungi, which show a distinct preference for NH4+ uptake (Plassard et al., 1991). Therefore, the first hypothesis of our study was supported, whereby mycorrhizal fungi switch the preferred N source uptake of their plant hosts, with the magnitude of uptake depending on the origin of the mycorrhizal fungi.
Since mycorrhizal fungi alter the N uptake patterns of C. goeringii seedlings in the top soil, it is logical to assume that similar uptake patterns occurred in the deeper soil layer; however, we did not observe a similar pattern at both soil depths. In contrast, C. goeringii seedlings inoculated with the fungal strains CLB113 and MLX102 exhibited a strong uptake of glycine, whereas those inoculated with CLB113 preferentially took up NO3− and NH4+. The reasons for this disparity in N uptake in the different soil layers might be related to a possible differentiation in mycorrhizal hyphae of the fungal partners of C. goeringii. As a result, our second hypothesis was only partly confirmed, whereby the extraradical mycelium facilitates the effective uptake of inorganic and organic N from deeper soil by the orchid, despite the presence of fewer roots in this layer.
In summary, non-mycorrhizal C. goeringii seedlings preferentially took up NO3− from tracers injected at 2·5-cm soil depth, while C. goeringii seedlings inoculated with all three mycorrhizas showed a strong preference for NH4+ uptake from tracers injected at 2·5-cm soil depth. This difference indicates that mycorrhizal fungi switch the N source uptake of C. goeringii from NO3− to NH4+. CLB113 and MLX102 strains were isolated from different Cymbidium species, but showed a similar N uptake pattern in the tracers injected at 2·5-cm soil depth. Hence, the disparity in N uptake in the different soil layers might be ascribed to the possible differentiation of mycorrhizal hyphae of the fungal partners of C. goeringii.
ACKNOWLEDGEMENTS
We thank Mr Rick Zenn and Dr Richard O'Hanlon very much for their great help with language improvements as well as constructive comments on this manuscript. We also thank two anonymous reviewers for their helpful suggestions which helped enhance this paper. This research was financially supported by the National Natural Science Foundation of China (30671717 and 31260175), key projects in the National Science and Technology Pillar Program during the Eleventh Five-Year Plan Period (2008BAC39B05), Southwest Forestry University, key research fund (BC2010FK01) and the Innovative Research Team and key disciplines of Forest Protection of Yunnan Universities (XKZ200905).
LITERATURE CITED
- Agerer R. Exploration types of ectomycorrhizae: a proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza. 2001;11:107–114. [Google Scholar]
- Alexander C, Alexander IJ, Hadley G. Phosphate uptake by Goodyera repens in relation to mycorrhizal infection. New Phytologist. 1984;97:391–400. [Google Scholar]
- Alexander I. A knight of symbiosis. New Phytologist. 2007;176:499–510. doi: 10.1111/j.1469-8137.2007.02259.x. [DOI] [PubMed] [Google Scholar]
- Berg-Pana H. Handbuch der orchideen-namen. Dictionary of orchid names. Dizionario dei nomi delle orchidee. Stuttgart: Ulmer; 2005. [Google Scholar]
- Cameron DD, Leake JR, Read DJ. Mutualistic mycorrhizal in orchids: evidence from plant–fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytologist. 2006;171:405–416. doi: 10.1111/j.1469-8137.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- Cameron DD, Johnson I, Leake JR, Read DJ. Mycorrhizal acquisition of inorganic phosphorus by the green-leaved terrestrial orchid Goodyera repens. Annals of Botany. 2007;99:831–834. doi: 10.1093/aob/mcm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS, III, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a nonmycorrhizal arctic sedge. Nature. 1993;361:150–153. [Google Scholar]
- Currah RS, Zelmer CD, Hambleton S, Richardson KA. Fungi from orchid mycorrhizas. In: Arditti J, Pridgeon AM, editors. Orchid biology: reviews and perspectives. Dordrech: Kluwer Academic Publishers; 1997. pp. 117–170. [Google Scholar]
- DuPuy D, Cribb P. Portland, OR: Timber Press; 1988. Genus Cymbidium. [Google Scholar]
- DuPuy D, Cribb P. Richmond: Kew Publishing; 2007. The genus Cymbidium. [Google Scholar]
- Finlay RD. Action and interaction in the mycorrhizal hyphosphere: a re-evaluation of the role of mycorrhizal symbiosis in nutrient acquisition and plant ecology. In: BassiriRad H, editor. Nutrient acquisition by plants: an ecological perspective. Heidelberg: Springer-Verlag; 2005. pp. 221–276. [Google Scholar]
- George E, Marschner H, Jakobsen I. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from the soil. Critical Review in Biotechnology. 1995;15:257–270. [Google Scholar]
- Hodge A, Fitter AH. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proceedings of the National Academy of Sciences of the USA. 2010;107:13754–13759. doi: 10.1073/pnas.1005874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof S. Arbuscular, ecto-related, orchid mycorrhizas – three independent structural lineages towards mycoheterotrophy: implications for classification? Mycorrhiza. 2009;19:357–363. doi: 10.1007/s00572-009-0240-7. [DOI] [PubMed] [Google Scholar]
- Leake JR. The biology of myco-heterotrophic (saprophytic) plants. New Phytologist. 1994;127:171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- Majerowicz N, Kerbauy GB. Effects of nitrogen forms on dry matter partitioning and nitrogen metabolism in two contrasting genotypes of Catasetum fimbriatum (Orchidaceae) Environmental and Experimental Botany. 2002;47:249–258. doi: 10.1016/s0098-8472(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Majerowicz N, Kerbauy GB, Nievola CC, Suzuki RM. Growth and nitrogen metabolism of Catasetum fimbriatum (Orchidaceae) grown with different nitrogen sources. Environmental and Experimental Botany. 2000;44:195–206. doi: 10.1016/s0098-8472(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Miransari M. Arbuscular mycorrhizal fungi and nitrogen uptake. Archives of Microbiology. 2011;193:77–81. doi: 10.1007/s00203-010-0657-6. [DOI] [PubMed] [Google Scholar]
- Neumann E, George E. Nutrient uptake: the arbuscular mycorrhiza fungal symbiosis as a plant nutrient acquisition strategy. In: Koltai H, Kapulnik Y, editors. Arbuscular mycorrhizas: physiology and function. Berlin: Springer Science+Business Media; 2010. [Google Scholar]
- Plassard C, Scheromm P, Mousain D, Salsac L. Assimilation of mineral nitrogen and ion balance in the two partners of ectomycorrhizal symbiosis: data and hypothesis. Experientia. 1991;47:340–349. [Google Scholar]
- Raab TK, Lipson DA, Monson RK. Non-mycorrhizal uptake of amino acids by roots of the alpine sedge Kobresia myosuroides: implications for the alpine nitrogen cycle. Oecologia. 1996;108:488–494. doi: 10.1007/BF00333725. [DOI] [PubMed] [Google Scholar]
- Rasmussen HN. Terrestrial orchids: from seed to mycotrophic plant. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Rasmussen HN, Rasmussen FN. Orchid mycorrhiza: implications of a mycophagous life style. Oikos. 2009;118:334–345. [Google Scholar]
- Read DJ. Mycorrhizas in ecosystems. Cellular and Molecular Life Sciences. 1991;47:376–391. [Google Scholar]
- Read DJ, Perez-Moreno J. Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytologist. 2003;157:475–492. doi: 10.1046/j.1469-8137.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- Rothstein DE. Soil amino-acid availability across a temperate-forest fertility gradient. Biogeochemistry. 2009;92:201–215. [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- Smith SE, Read D. Mycorrhizal symbioses. London: Academic Press; 2008. [Google Scholar]
- Sneh B, Burpee L, Ogoshi A. St Paul, MN: APS Press; 1991. Identification of Rhizoctonia species. [Google Scholar]
- Thornton B. Uptake of glycine by non-mycorrhizal Lolium perenne. Journal of Experimental Botany. 2001;52:1315–1322. [PubMed] [Google Scholar]
- Trépanier M, Lamy M-P. Phalaenopsis can absorb directly through their roots. Plant and Soil. 2009;319:95–100. [Google Scholar]
- Veresoglou SD, Chen B, Rillig MC. Arbuscular mycorrhiza and soil nitrogen cycling. Soil Biology and Biochemistry. 2012;46:53–62. [Google Scholar]
- Wang Y-T. High NO3-N to NH4-N ratios promote growth and flowering of a hybrid Phalaenopsis grown in two root substrates. Hortscience. 2008;43:350–353. [Google Scholar]
- Warcup JH, Talbot PHB. Perfect states of Rhizoctonia associated with orchids. New Phytologist. 1967;66:631–641. [Google Scholar]
- Wu JR, Ma HC, Lü M, et al. Rhizoctonia fungi enhance the growth of the endangered orchid Cymbidium goeringii. Botany. 2010;88:20–29. [Google Scholar]