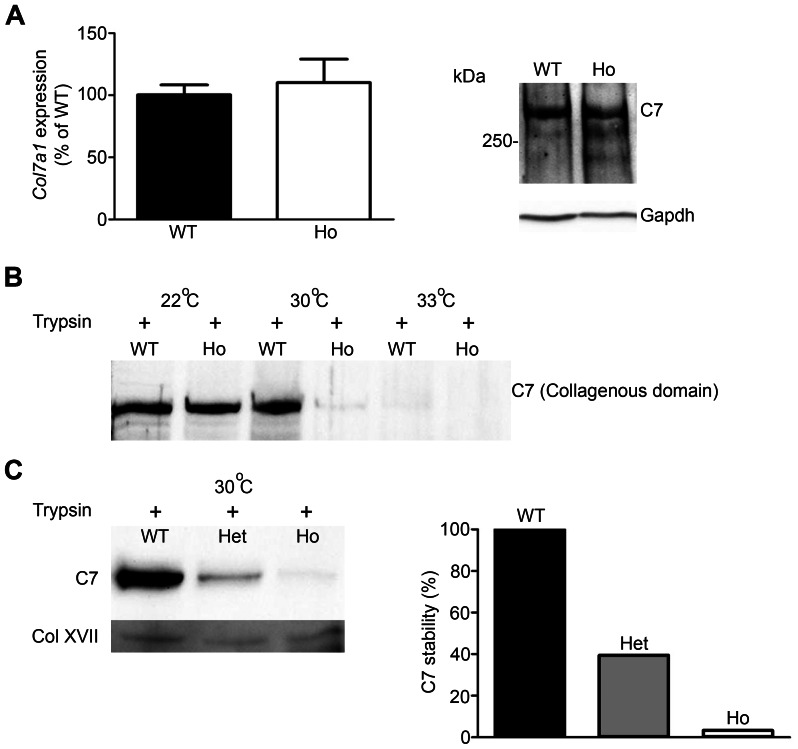
Figure 5. The p.G1867D mutation impacts protein stability.
A, C7 expression in cultured fibroblasts from wild-type and p.G1867D homozygous rats. qPCR analysis (left panel) and Western blot (right panel) demonstrate no notable change in mRNA or protein expression. B, Limited trypsin digestion reveals greatly reduced thermal stability of mutant C7, as shown by Western blot detecting the intact collagenous domain of C7. Mutant C7 containing the p.G1867D substitution was degraded at lower temperature than wild-type C7, indicating a reduction in thermal stability due to faulty triple helix folding of the collagenous domain. WT = wild-type; Ho = homozygous for p.G1867D. C, Limited trypsin digestion of C7 extracted from wild-type, heterozygous and homozygous keratinocytes. The lysates were heated to 30°C and allowed to cool down to room temperature before limited trypsin digestion. Western blot detecting the intact collagenous domain of C7 (left panel) shows reduced stability of heterozygous molecules and loss of stability of homozygous molecules. The right panel shows densitometric quantification of the collagenous domain of C7 and normalization to collagen XVII, which was used as a loading control. The value of wild-type C7 was set to 100% and the percent stability, as indicated by resistance to trypsin digestion, of heterozygous and homozygous C7 was calculated in relation to this value. WT = wild-type; Het = heterozygous for p.G1867D; Ho = homozygous for p.G1867D.